Preparation method of stable inorganic hydrated salt-based phase change heat storage material
A phase change heat storage material, inorganic hydrated salt technology, applied in heat exchange materials, chemical instruments and methods, etc., can solve the problems of reduced efficiency and phase change material precipitation, and achieves good performance, low corrosion rate, and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
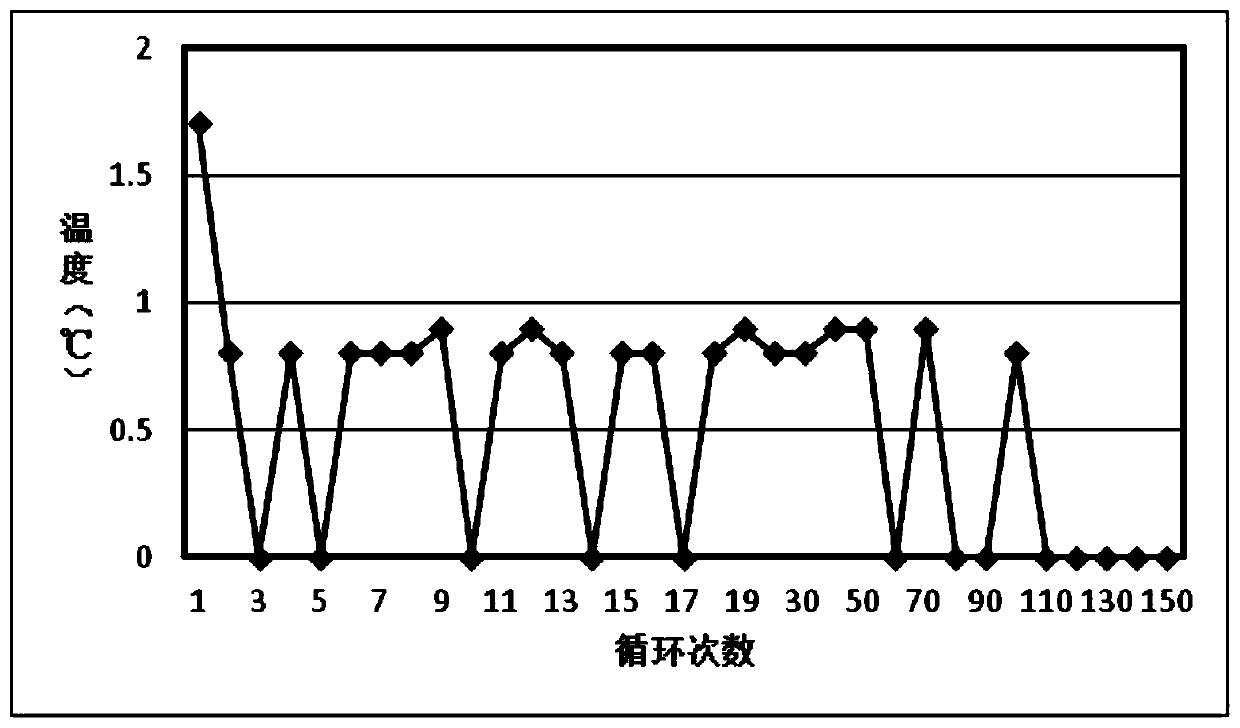
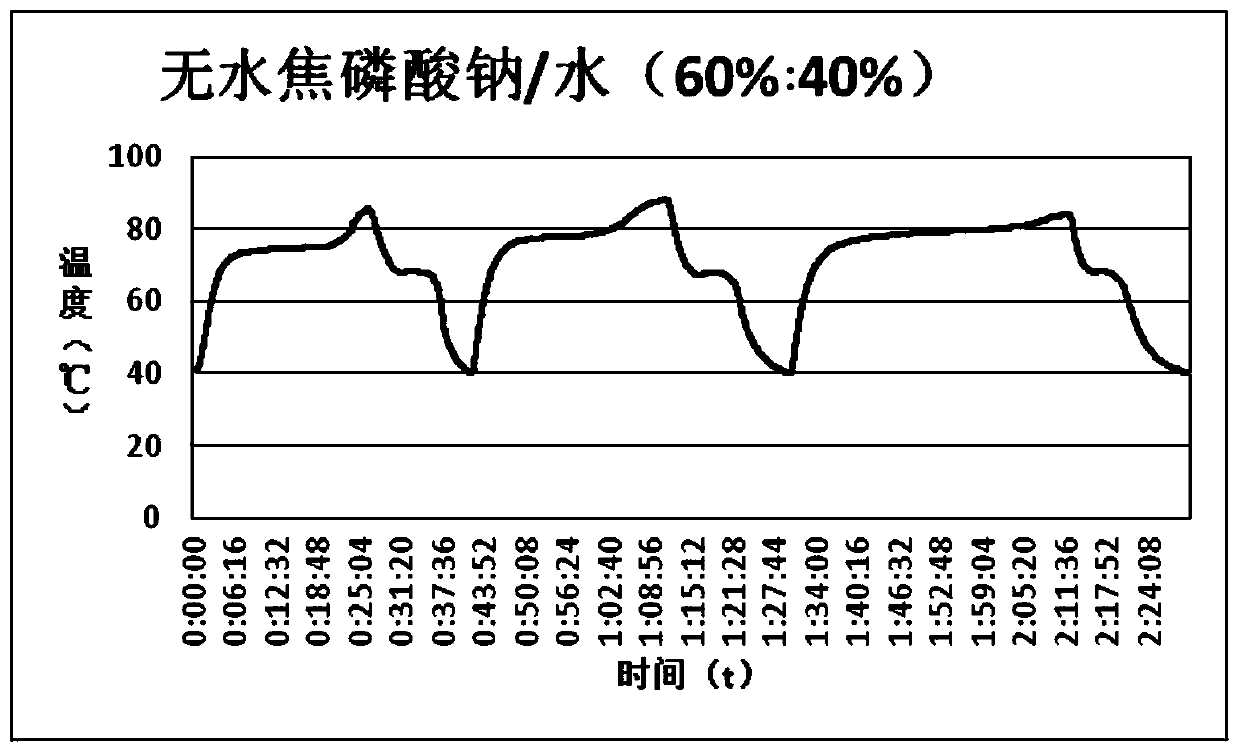
[0024] This example provides a stable inorganic hydrated salt-based phase change heat storage material, which is composed of anhydrous sodium pyrophosphate and water according to the mass percentage of 60:40. The preparation method is as follows: grind anhydrous sodium pyrophosphate to When the diameter of the particles is less than 50 mesh, add water and stir and place in a water bath at 80°C for heating. After the solid is completely melted into a liquid, shake it evenly and pour it into the container. The volume of the injected container is controlled at 90%, and then cool to room temperature and seal the container. . The obtained phase change heat storage material was tested for cycle stability, and 150 times of charge and discharge cycle tests were carried out. The test results are as follows: figure 1 , 2 As shown, the experiment shows that the plateau temperature of phase change material crystallization is 68.2°C, and the relative undercooling degree is 0.8°C. The mate...
Embodiment 2
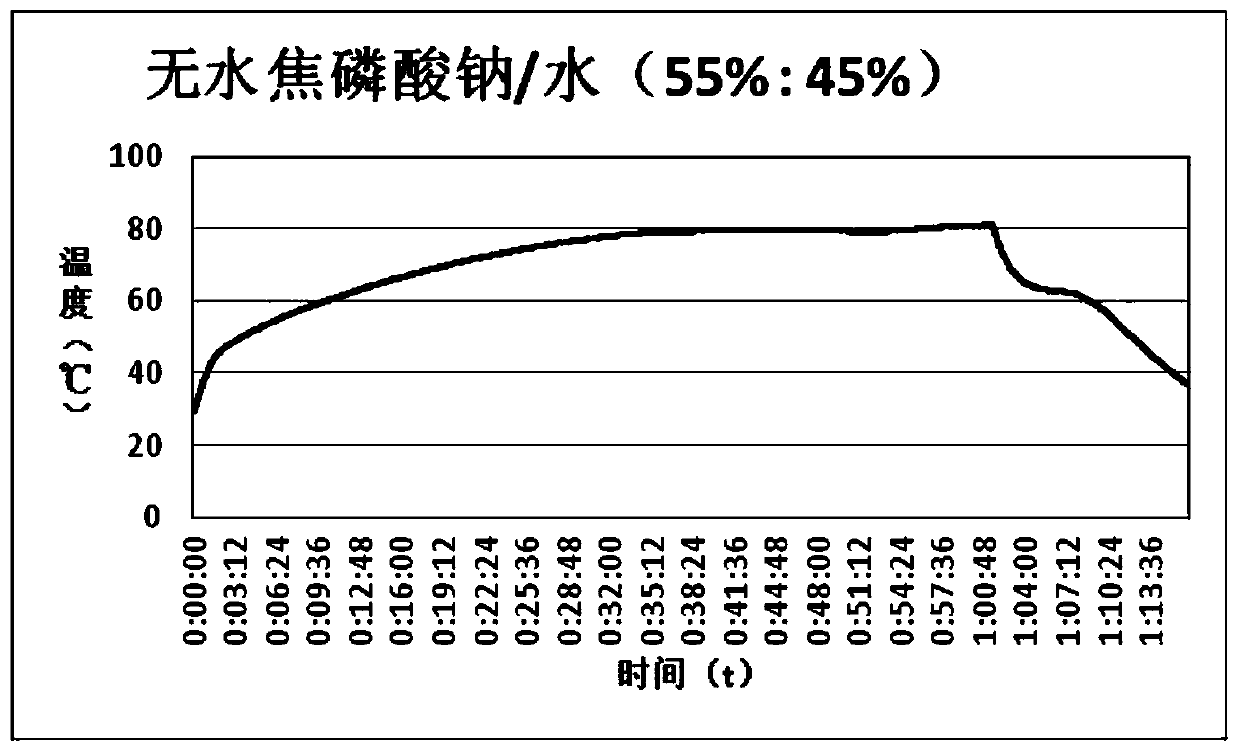
[0026] The preparation method of this example is the same as that of Example 1, except that in the phase change heat storage material, the mass percentage of anhydrous sodium pyrophosphate and water is 55:45. Experiments show that the plateau temperature of phase change material crystallization is 62.8°C, as image 3 Shown; its relative subcooling is 1°C.
Embodiment 3
[0028] The preparation method of this example is the same as that of Example 1, except that in the phase change heat storage material, the mass percentage of anhydrous sodium pyrophosphate and water is 65:35. Experiments show that the plateau temperature of phase change material crystallization is 63.5°C, as Figure 4 Shown; its relative subcooling is 1°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com