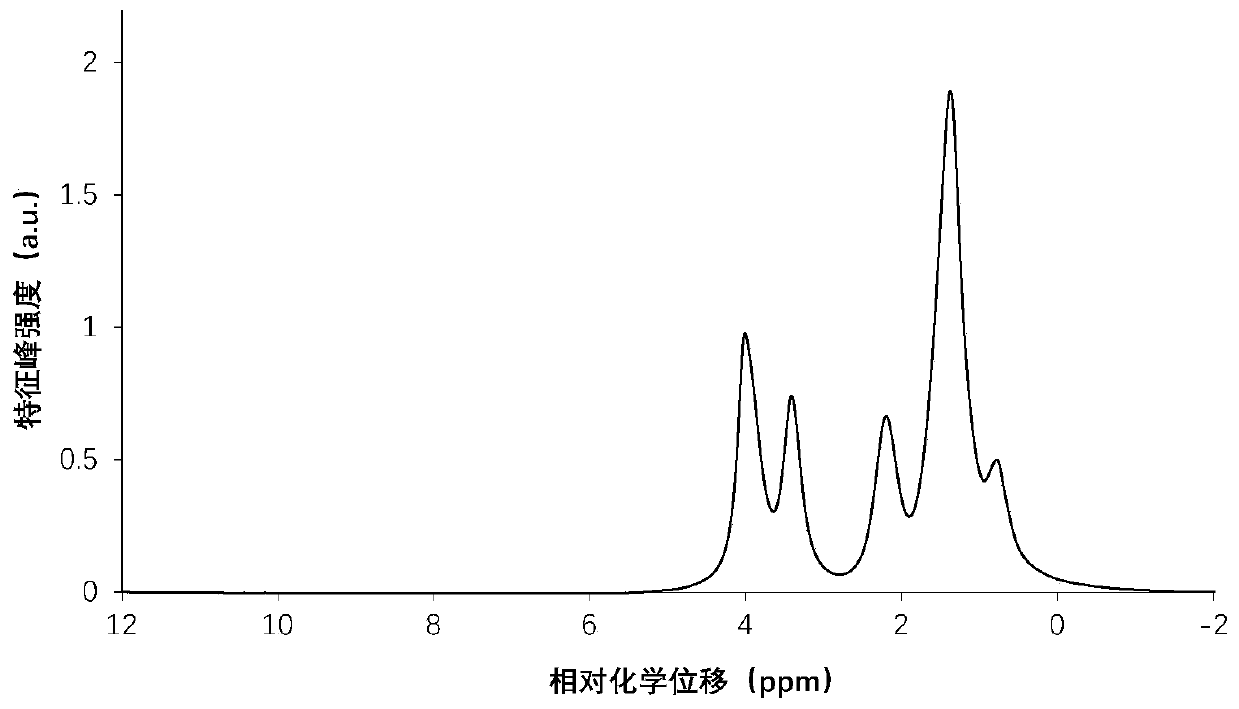
Method for measuring hydroxyl value of polycaprolactone triol by utilizing 1H nuclear magnetic resonance spectra
A technology of hydrogen nuclear magnetic resonance spectroscopy and polycaprolactone, which is applied in the fields of nuclear magnetic resonance analysis, climate sustainability, water resources assessment, etc., can solve the problem of rapid detection of hydroxyl value of polycaprolactone polyols that has not been seen , low analysis efficiency, high temperature requirements, etc., to achieve good environmental protection benefits, improve analysis efficiency, and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
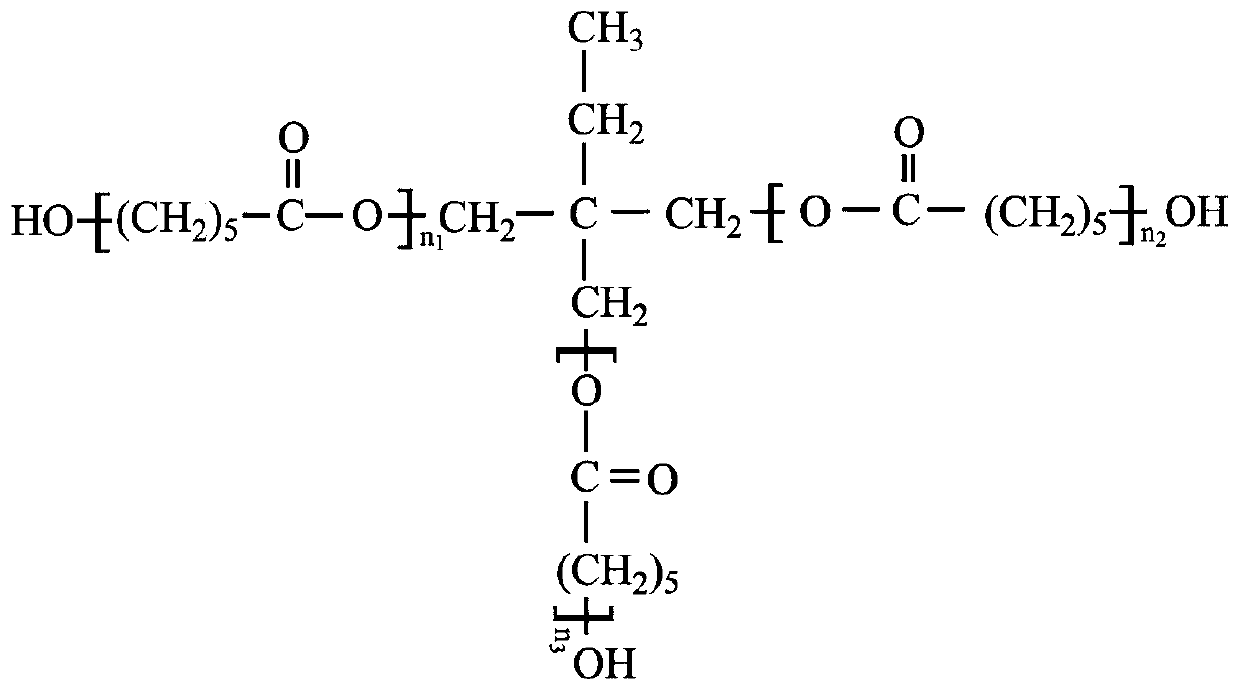
[0033] Analysis of hydroxyl value of polycaprolactone triol (sample 1)
[0034] 1. Sample Analysis
[0035] Taking the polycaprolactone triol (sample 1) of a domestic chemical company as an example, the calculation process of the hydroxyl value of the polycaprolactone triol sample is explained. The steps are as follows:
[0036] (1) Add the analysis sample to the NMR tube.
[0037](2) Place the NMR tube in the NMR analyzer, select the corresponding analysis plan, scan the sample, and obtain the H NMR spectrum of the sample;
[0038] (3) Integrate the characteristic peak areas of the five regions of -0.55~0.93ppm, 0.93~1.89ppm, 1.89~2.79ppm, 2.79~3.64ppm, 3.64~5.11ppm in the sample hydrogen nuclear magnetic resonance spectrum, and calculate and obtain -0.55~ The total area of three characteristic peaks in the 2.79ppm region X 1 is 67.190, the total area of two characteristic peaks in the region of 2.79~5.11ppm X 2 is 31.763, then the ratio of the total number of H atoms...
Embodiment 2
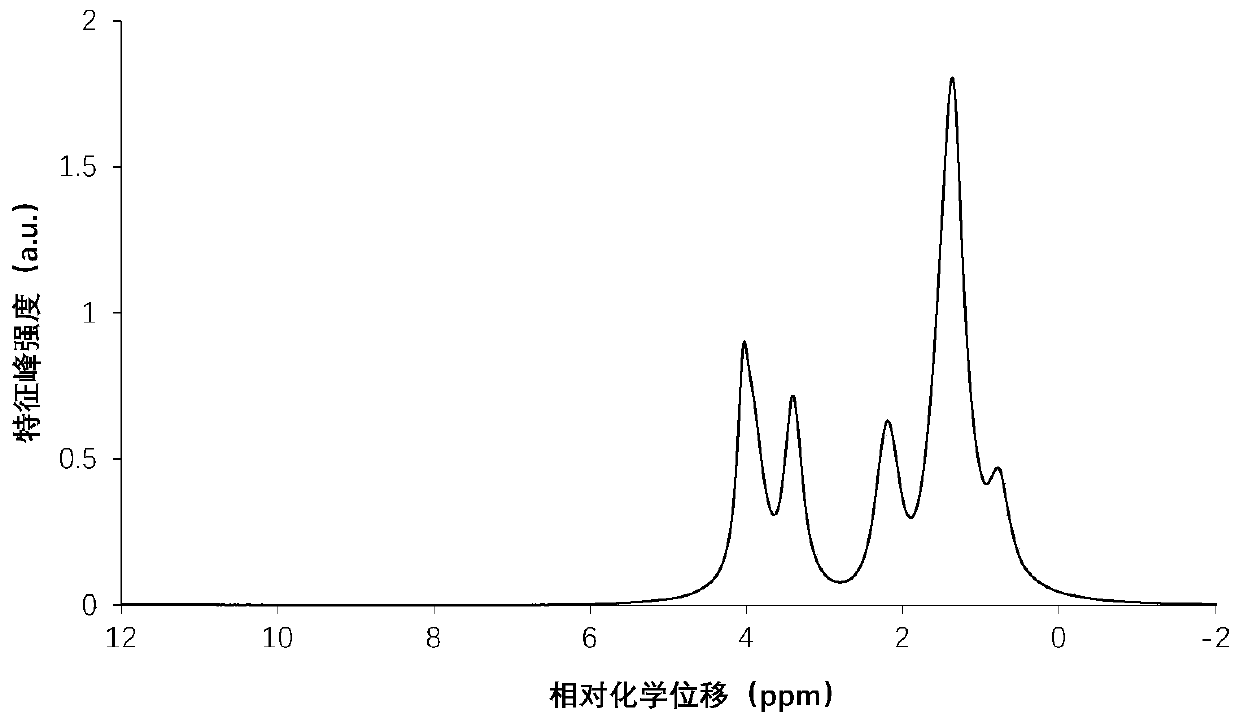
[0045] Analysis of hydroxyl value of polycaprolactone triol (sample 2)
[0046] 1. Sample Analysis
[0047] Select polycaprolactone triol (sample 2), another domestic chemical company, to analyze the hydroxyl value of its polycaprolactone triol sample, the steps are as follows:
[0048] (1) Add the analysis sample to the NMR tube.
[0049] (2) Place the NMR tube in the NMR analyzer, select the corresponding analysis plan, scan the sample, and obtain the H NMR spectrum of the sample;
[0050] (3) Integrate the characteristic peak areas of the five regions of -0.55~0.93ppm, 0.93~1.89ppm, 1.89~2.79ppm, 2.79~3.64ppm, 3.64~5.11ppm in the sample hydrogen nuclear magnetic resonance spectrum, and calculate and obtain -0.55~ The total area of three characteristic peaks in the 2.79ppm region X 1 is 69.267, the total area of two characteristic peaks in the region of 2.79~5.11ppm X 2 is 31.543, then the ratio of the total number of H atoms in each structure in the two regions is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com