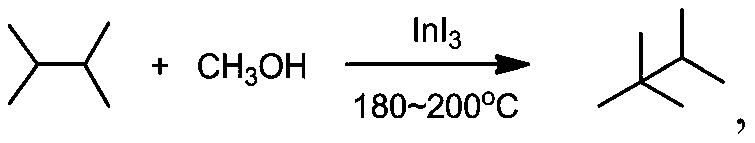Preparation method of branched alkane
A branched-chain alkane and alkyl alcohol technology, which is applied in the field of branched-chain alkane preparation, can solve the problems of inability to recycle, high equipment requirements, and large dosage, and meets the requirements of less by-products, mild reaction conditions, and equipment corrosion resistance. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The present invention provides a method for preparing branched alkanes, including the following steps:
[0025] Under protective atmosphere, mixing pinacolone, metal alkyl reagent and organic solvent, and then carry out alkylation reaction to obtain alkyl alcohol intermediate;
[0026] Under the protective atmosphere, the alkyl alcohol intermediate, the alkyl silane, the acid catalyst and the organic solvent are mixed and then subjected to elimination reduction reaction to obtain branched alkanes.
[0027] In the present invention, under the protective atmosphere, the pinacolone, the alkyl metal reagent and the organic solvent are mixed and then the alkylation reaction is carried out to obtain the alkyl alcohol intermediate. In the present invention, the alkyl metal reagent preferably includes alkyl lithium or alkyl Grignard reagent; the alkyl group in the alkyl metal reagent preferably includes methyl, ethyl, propyl or butyl; specifically, The alkyl metal reagent preferably ...
Embodiment 1
[0041] (1) Synthesis of 2,3,3-trimethylbutanol
[0042] Install a dropping funnel and argon protection device on the jacketed four-neck flask, and use argon protection for the reaction system; add a diethoxymethane solution of pinacolone (from 0.225mol pinacolone and 75mL) to the dropping funnel Mix diethoxymethane), add methyllithium diethoxymethane solution (198mL, of which the concentration of methyllithium is 1.6mol / L) into a four-necked jacketed flask with a syringe, and cool to -10℃ , Add the diethoxymethane solution of pinacolone dropwise to the four-neck jacketed flask to control the temperature of the system to be lower than -5°C; after the dropwise addition, carry out the alkylation reaction for 6h ( During the reaction, samples were taken for GC detection, and the reaction process was monitored by detecting the conversion of raw materials and the formation of products); after the alkylation reaction was completed, saturated chlorination was added to the obtained alkyla...
Embodiment 2
[0049] (1) Synthesis of 2,3,3-Trimethylpentanol
[0050] Install a dropping funnel and argon protection device on the jacketed four-necked flask, and use argon protection for the reaction system; add pinacolone tetrahydrofuran solution (from 0.225mol pinacolone and 75mL tetrahydrofuran) into the dropping funnel , Add ethyl lithium tetrahydrofuran solution (250 mL, the concentration of ethyl lithium is 1.6 mol / L) into the four-necked jacketed flask with a syringe, cool to -10℃, and add pinnacle dropwise to the four-necked jacketed flask The tetrahydrofuran solution of ketone, control the temperature of the system to be lower than -5°C; after the addition is complete, carry out the alkylation reaction for 6h under stirring and holding at 0°C (sample during the reaction for GC detection, by detecting the conversion of raw materials and the formation of products If the situation, monitor the progress of the reaction); after the alkylation reaction is completed, add a saturated aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com