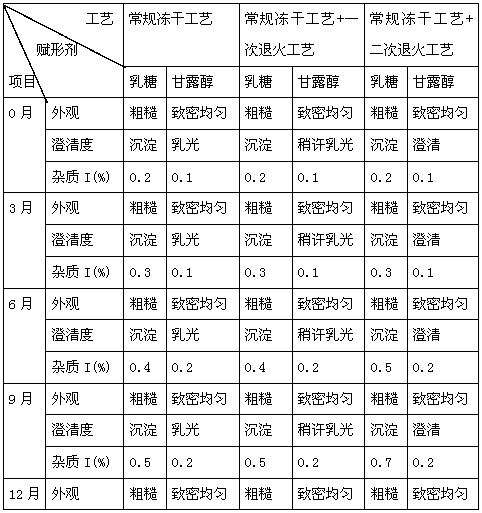
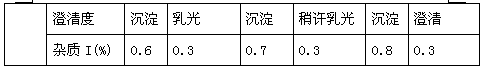
Neostigmine methylsulfate frozen-dried preparation for injection and preparation method of neostigmine methylsulfate frozen-dried preparation
A technology of neostigmine methylsulfate and freeze-dried preparation, applied in the field of medicine, can solve problems such as unfavorable product storage or long-distance transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] a. Weigh 1g neostigmine methylsulfate, 20g mannitol, dissolve and dilute to 1000ml with water for injection;
[0053] b. Filter with a 0.22um filter element, and fill 1ml of the filtrate into a 3ml vial;
[0054] c. Put the control bottle of sub-package liquid into the freeze dryer;
[0055] d. Reduce the shelf temperature of the freeze dryer to below -35°C at a rate of 30°C per hour;
[0056] e. When the temperature of the product drops below -40°C, keep it warm for 2 hours;
[0057] f. Raise the shelf temperature to -20°C and keep it warm for 1 hour;
[0058] g. Lower the shelf temperature to -40°C and keep it warm for 2 hours;
[0059] h. Raise the shelf temperature to -25°C and keep it warm for 1 hour;
[0060] i. Lower the shelf temperature to -45°C and keep it warm for 4 hours;
[0061] j. Start vacuuming, when the vacuum degree is below 10-20Pa, start to heat up;
[0062] k. Raise the shelf temperature to -15°C and keep it for 15 hours;
[0063] l. Raise the...
Embodiment 2
[0067] a. Weigh 1g neostigmine methylsulfate, 50g mannitol, dissolve and dilute to 1500ml with water for injection;
[0068] b. Filter with a 0.22um filter element, and fill 1.5ml of the filtrate into a 3ml vial;
[0069] c. Put the control bottle of sub-package liquid into the freeze dryer;
[0070] d. Reduce the shelf temperature of the freeze dryer to below -35°C at a rate of 30°C per hour;
[0071] e. When the temperature of the product drops below -40°C, keep it warm for 2 hours;
[0072] f. Raise the shelf temperature to -20°C and keep it warm for 2 hours;
[0073] g. Lower the shelf temperature to -40°C and keep it warm for 2 hours;
[0074] h. Raise the shelf temperature to -25°C and keep it warm for 2 hours;
[0075] i. Then lower the shelf temperature to -45°C and keep it warm for 5 hours;
[0076] j. Start vacuuming, when the vacuum degree is below 10-20Pa, start to heat up;
[0077] k. Raise the shelf temperature to -15°C and keep it for 17 hours;
[0078] l...
Embodiment 3
[0082] a. Weigh 1g neostigmine methosulfate, 100g mannitol, dissolve and dilute to 2000ml with water for injection;
[0083] b. Filter with a 0.22um filter element, and fill 2ml of the filtrate into a 5ml vial;
[0084] c. Put the control bottle of sub-package liquid into the freeze dryer;
[0085] d. Reduce the shelf temperature of the freeze dryer to below -35°C at a rate of 30°C per hour;
[0086] e. When the temperature of the product drops below -45°C, keep it warm for 3 hours;
[0087] f. Raise the shelf temperature to -20°C and keep it warm for 2 hours;
[0088] g. Lower the shelf temperature to -40°C and keep it warm for 3 hours;
[0089] h. Raise the shelf temperature to -25°C and keep it warm for 2 hours;
[0090] i. Then lower the shelf temperature to -50°C and keep it warm for 5 hours;
[0091] j. Start vacuuming, when the vacuum degree is below 10-20Pa, start to heat up;
[0092] k. Raise the shelf temperature to -15°C and keep it for 20 hours;
[0093] l. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com