an injection filler
A technology of fillers and copolymers, used in drug delivery, prostheses, microcapsules, etc., can solve the problems of high production management costs, low assurance of sterility of the final product, and difficulty in maintaining clinical application effects. The effect of softening deformation and prolonging the degradation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The technical solutions in the embodiments of the present invention will be clearly and completely described below. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
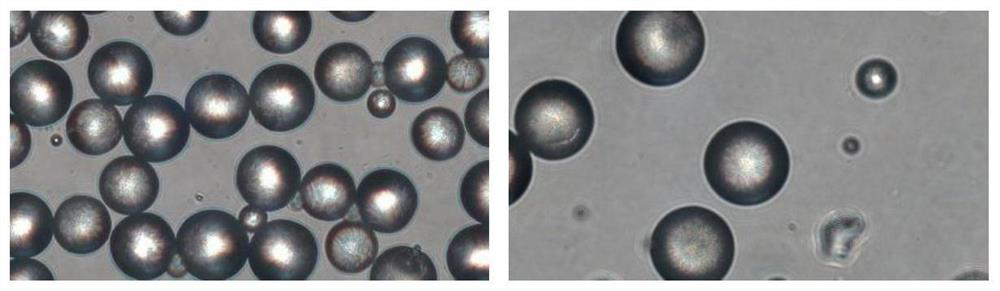
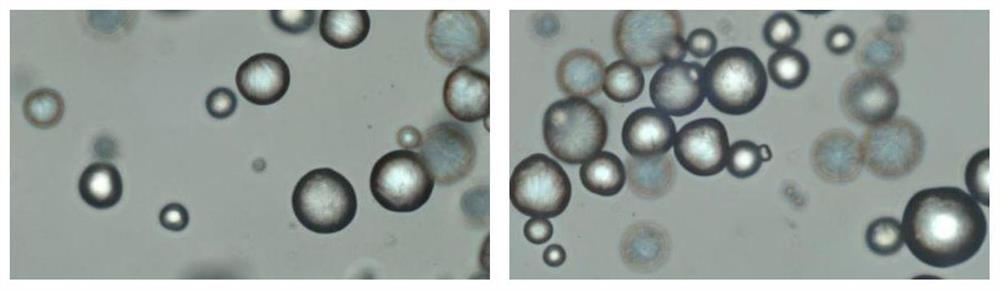
[0015] The invention provides an injection filler, which comprises the following components in percentage by weight: 1-50% absorbable synthetic polymer microspheres, 1-50% cross-linked sodium hyaluronate gel, 0.01-50% 5% surfactant and 10% to 90% neutral liquid.
[0016] Absorbable synthetic polymer microspheres are used to prolong the degradation time, selected from homopolymers or copolymers consisting of: PCL, PGA, PVA, PLA, PDO, P-TMC. Its weight percent content is preferably 10%-40%, most preferably 20%-35%.
[0017] The degr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com