Reagent kit for detecting human WT1 fusion genes, and application method of reagent kit
A technology that integrates genes and kits, and is applied in biochemical equipment and methods, and microbial measurement/inspection, etc. It can solve the problems of unsatisfactory FCM sensitivity and achieve the effects of improved specificity, stable concentration, and reduced pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
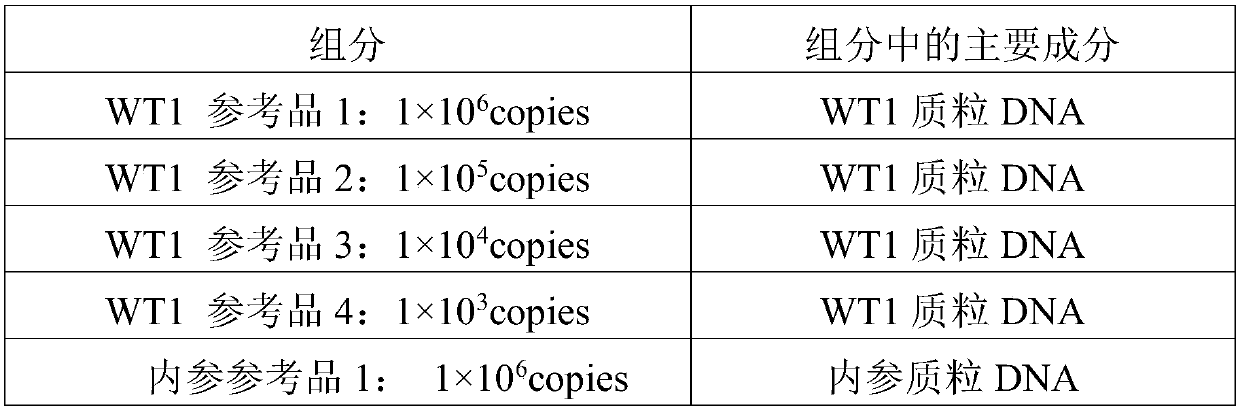
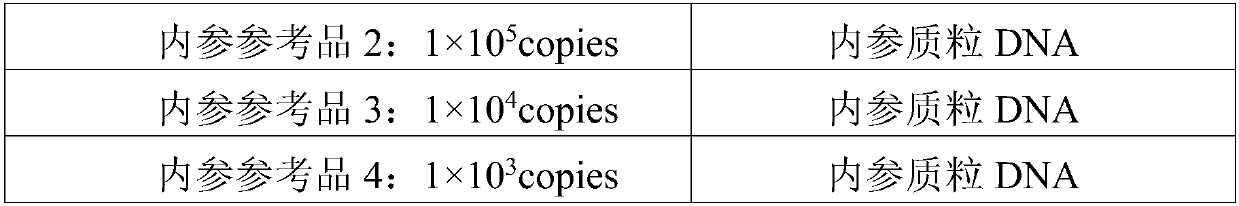
[0095] A test kit for detecting human WT1 fusion gene, the composition of which is shown in Table 5:
[0096] table 5:
[0097]
[0098]
[0099] Among them, the activity unit of UNG enzyme is 1U / μl, the activity unit of Taq enzyme is 5U / μl, and the activity unit of RT enzyme is 200U / μl.
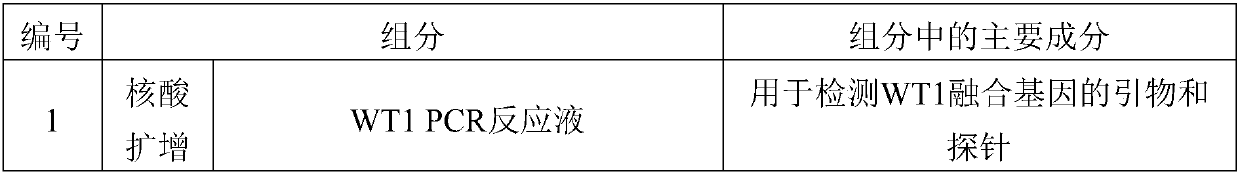
[0100] The packaging and content of each component of the kit are shown in Table 6:
[0101] Table 6:
[0102]
Embodiment 2
[0103] Example 2: Linear Detection
[0104] Select 100 μl each of the WT1 plasmid with a determined concentration and the plasmid of the internal reference gene to make a high-concentration DNA sample (L0). The L0 sample was serially diluted with a gradient of 1:10 to obtain two sets of linear quality control substances L1, L2, L3 and L4, respectively. The specific composition is shown in Table 9 and Table 10.
[0105] The logarithmic value of the concentration is Y, and the mean value of CT is X, and linear fitting is carried out to calculate the linear correlation coefficient r. The specific experimental results are shown in Table 7 and Table 8:
[0106] Table 7
[0107] WT1 Linear Quality Control Enter the number of reaction templates (copies) WT1-L0 5×10 6
[0108] Table 8
[0109] Internal reference gene linear quality control Enter the number of reaction templates (copies) Internal reference-L0 5×10 6
[0110] Operate according t...
Embodiment 3
[0111] Example 3: Detection of the accuracy of the kit of the present application
[0112] Adopt the test kit of embodiment 1 to detect the accuracy reference product: wherein, the preparation method of the accuracy reference product is as shown in Table 9:
[0113] Table 9
[0114]
[0115]
[0116] The prepared accuracy reference product is detected, and the experimental results obtained are shown in Table 10:
[0117] Table 10
[0118]
[0119] Using the test kit of the present application to detect the accuracy reference product, the positive coincidence rate is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com