Application of combined matrix dhb/dhbh in detection of reducing sugar in maldi mass spectrometry
A combined, mixed-matrix technology, applied in the field of sugar biochemical analysis, can solve the problems of Schiff base instability, limit wide application, sample signal suppression, etc., to improve crystallization uniformity, improve detection sensitivity, improve accuracy and stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
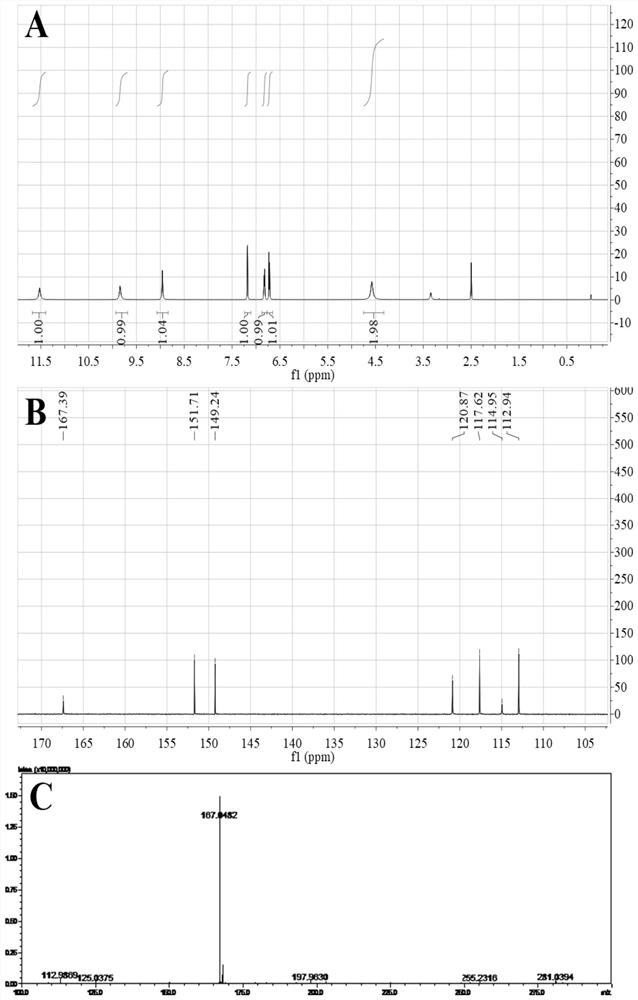
[0077] Example 1: Synthesis and characterization of DHBH
[0078] 1. Synthesis of DHBH
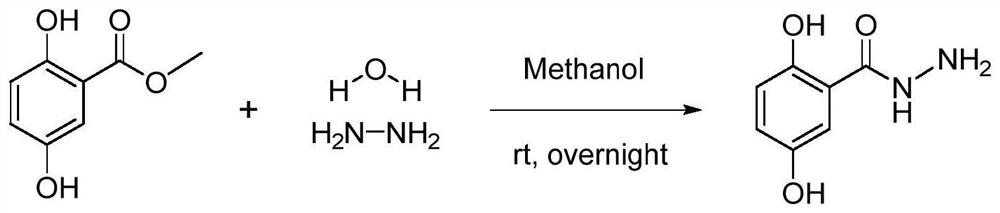
[0079] Add 0.168 g (0.001 mol) of methyl 2,5-dihydroxybenzoate and 10 mL of methanol in turn into a round bottom flask, stir at room temperature for 5 min and mix well. Then slowly add 0.2 mL (about 0.004 mol) of 85% hydrazine hydrate solution dropwise, and stir overnight at room temperature at 25° C. until the raw materials react completely to form a brown precipitate.
[0080] 2. DHBH separation and purification
[0081] The excess solvent and hydrazine hydrate were removed by rotary evaporation at room temperature 25°C to obtain a viscous solid. Then, excess methanol and 1 mL deionized water were added to redissolve the product, and it was recrystallized using a rotary evaporator. The temperature was controlled at room temperature 25°C, and the speed of the solvent rotary evaporation was slowed down until about 3 mL of solvent remained in the flask. The precipitate was washed thre...
Embodiment 2
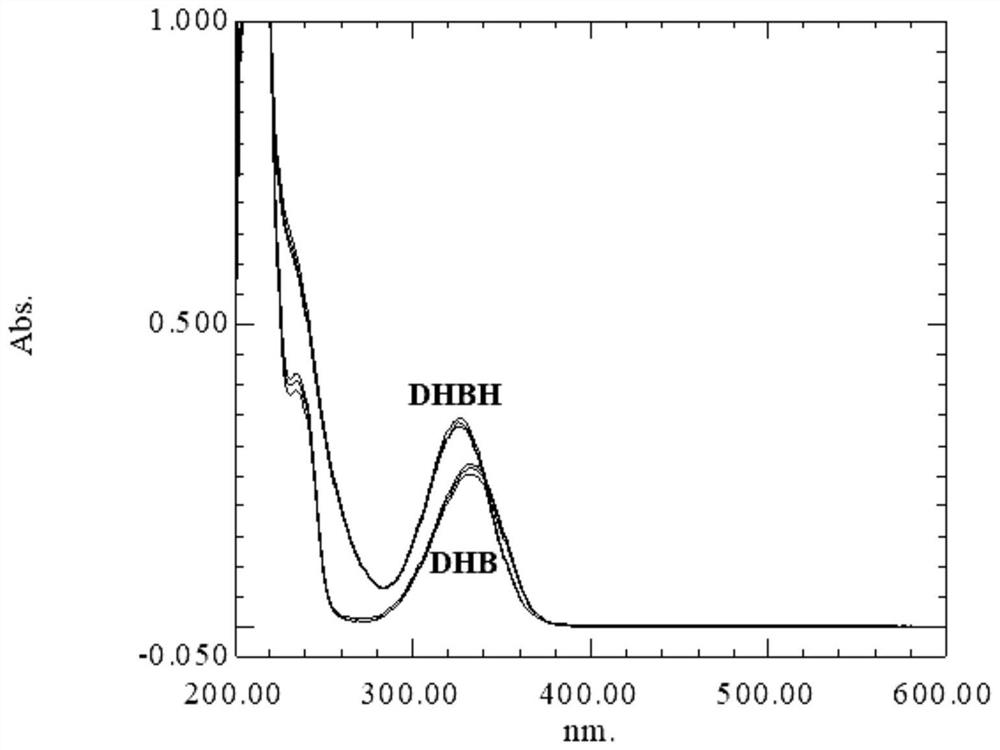
[0085] Example 2: DHBH itself is used as a MALDI matrix to detect carbohydrates
[0086] (1) Prepare 20pmol / μL G6 and 0.1mg / mL D2000 sugar solution, which can be stored in a refrigerator at 4°C;
[0087] (2) Prepare the DHBH methanol solution in 0.1mol / L embodiment 1, which can be stored in a refrigerator at 4°C;
[0088] (3) Prepare DHB methanol solutions of 0.01, 0.025, 0.05, 0.1, 0.2, 0.3 and 0.4mol / L respectively, which can be stored in a refrigerator at 4°C;
[0089] (4) Take 1 μL of the G6 sugar solution or D2000 sugar solution in step 1 and 1 μL of the DHBH methanol solution in step 2, respectively, and place them on the same well of the polished steel target plate in sequence. Use a pipette gun to pipette directly onto the MALDI target plate 10 times for mixing. Dry naturally at 15°C;
[0090] (5) As a control, take 1 μL of the G6 sugar solution or D2000 sugar solution in step 1 and 1 μL of the 0.1 mol / L DHB methanol solution in step 2, respectively, and spot on the...
Embodiment 3
[0093] Example 3: Combining DHBH and DHB to build a reducing sugar target plate derivatization strategy
[0094] 1. Optimization of the molar concentration ratio of organic acid matrix and DHBH in the mixed matrix
[0095] (1) Prepare DHB / DHBH mixed matrix, use DHB methanol solutions with different concentrations prepared in Example 2 as solvent and DHBH as solute, prepare 0.1M DHBH mixed solutions respectively. That is, the DHB / DHBH mixed matrix solution of 0.01:0.1, 0.025:0.1, 0.05:0.1, 0.1:0.1, 0.2:0.1, 0.3:0.1, 0.4:0.1;
[0096] (2) Prepare CHCA methanol solutions of 0.1, 0.05, 0.025, and 0.01M respectively, which can be stored in a refrigerator at 4°C;
[0097] (3) Prepare CHCA / DHBH mixed matrix, the CHCA / DHBH mixed matrix of 0.01:0.1, 0.025:0.1, 0.05:0.1 and 0.1:0.1 can adopt the preparation method in step (1), that is, use different concentrations in step (2) CHCA methanol solution was used as the solvent, DHBH was used as the solute, and 0.1M DHBH mixed solutions w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com