Compound, preparation method and application in preparation of GSK-3beta inhibitor
A compound and composition technology, applied in the field of medicine, can solve the problems of limited drug effect, lack of Aβ aggregation and depolymerization, complex pathogenesis, etc., and achieve the effect of novel structure, reducing toxic side effects, and promoting depolymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: Synthesis of the compound of formula I.
[0070] (1) Dissolve compound 1 (5.56g, 12mmol) and compound 2 (4.11g, 14.4mmol) in dioxane (16mL) and water (0.8mL), add Pd(dppf)Cl 2 (0.35g, 0.48mmol), and K 2 CO 3 (4.38g, 36mmol), under the protection of nitrogen, stirred at 100°C for 2.5h. Filter while hot and concentrate under reduced pressure. A small amount of water was added to the residue and extracted with dichloromethane. After the organic phases were combined, they were washed with water and saturated brine, and dried over anhydrous sodium sulfate. Filtration and concentration under reduced pressure gave the crude product as off-white solid.
[0071] The above crude product was dissolved in 60 mL of methanol, 20 mL of potassium hydroxide (6.8 g, 120 mmol) aqueous solution was added, and the reaction was stirred at 60° C. for 1.5 h. Cool to room temperature, adjust pH to neutral with saturated potassium bisulfate solution, and extract with ethyl ac...
Embodiment 2
[0076] Example 2: GSK-3 inhibitory activity experiment.
[0077] 1. Test method:
[0078] Incubate the FAM-labeled substrate with kinase, ATP and a certain concentration of compound (from Example 1) solution at 28°C for 1 h. After quenching with a quencher, use a Caliper instrument to measure the conversion rate of the substrate. The better the inhibition, the lower the conversion value. The results are shown in Table 1.
[0079] 2. Test results:
[0080] The compound of formula I prepared in Table 1 Example 1 has inhibitory activity on GSK-3β
[0081] compound GSK-3β% inhibition rate a
[0082] a Indicates the inhibition rate of different subtypes of GSK-3 when the concentration is 1 μM; b half effective inhibitory concentration.
Embodiment 3
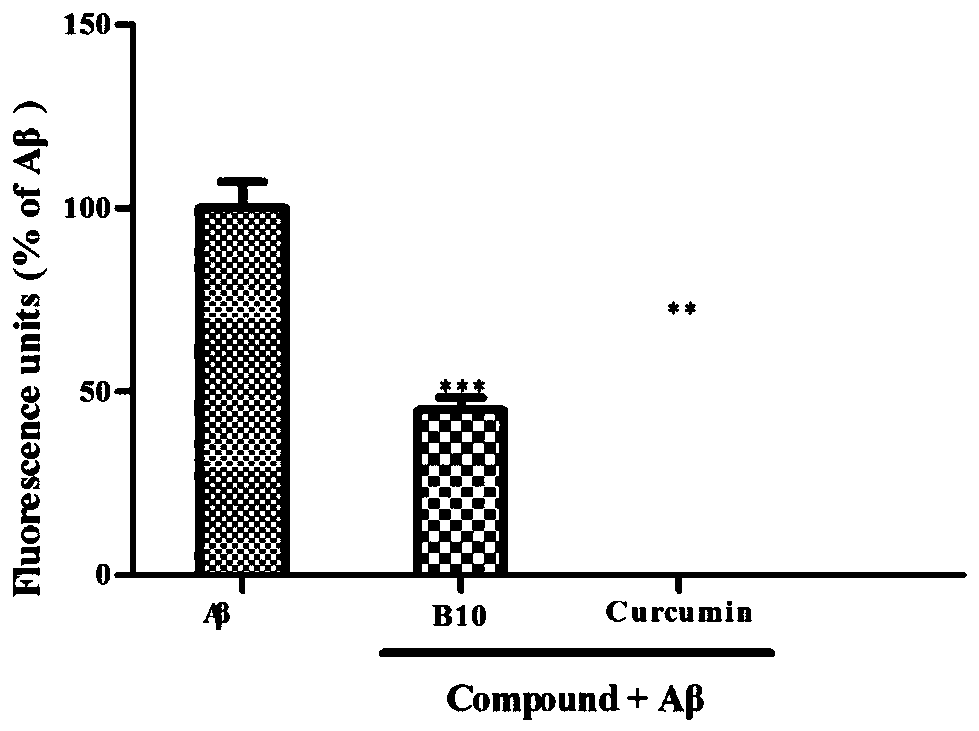
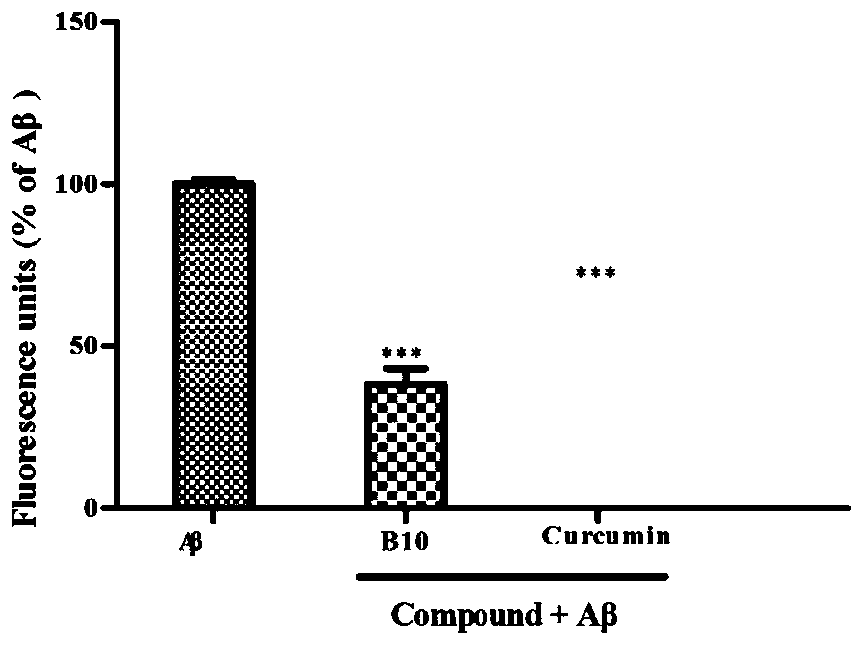
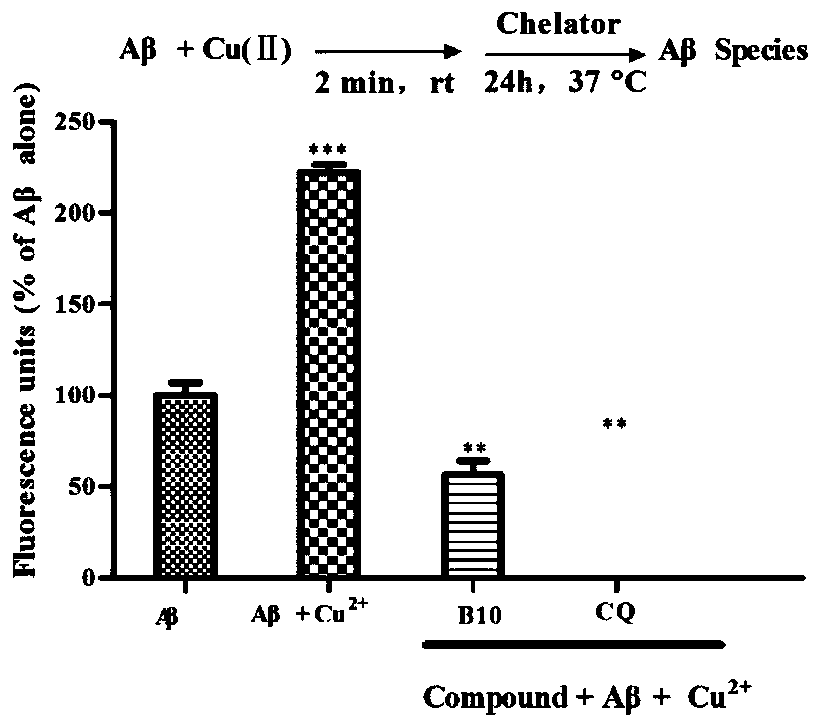
[0083] Example 3: Aβ aggregation inhibition experiment.
[0084] 1. Test method:
[0085] 0.5 mg of hexafluoroisopropanol-treated Aβ 1-42 Dissolved in dimethyl sulfoxide (DMSO) to make 2mM stock solution, aliquoted and stored at -20°C for future use. The stock solution was diluted to 40 μM with 50 mM PBS buffer (pH=7.4, 100 mM NaCl). The compound of formula I (B10) and the positive control drug Curcumin (Curcumin) were each dissolved in DMSO to form a 2mM stock solution, and the stock solution was diluted to a concentration of 40 μM with 50 mM PBS buffer (pH=7.4, 100 mM NaCl) for use. Aβ 1-42 (20 μL, 40 μM) and 20 μL PBS buffer, Aβ 1-42 (20 μ L, 40 μ M) and formula I compound (20 μ L, 40 μ M), Aβ 1-42 (20 μL, 40 μM) and Curcumin (20 μL, 40 μM) were respectively placed in a 96-well black plate and incubated at 37° C. for 24 h. Add 160 μL of thioflavin T glycine-sodium hydroxide (50 mM, pH=8.0) buffer solution with a concentration of 5 μM into the reaction well, place it i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com