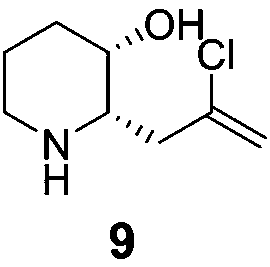
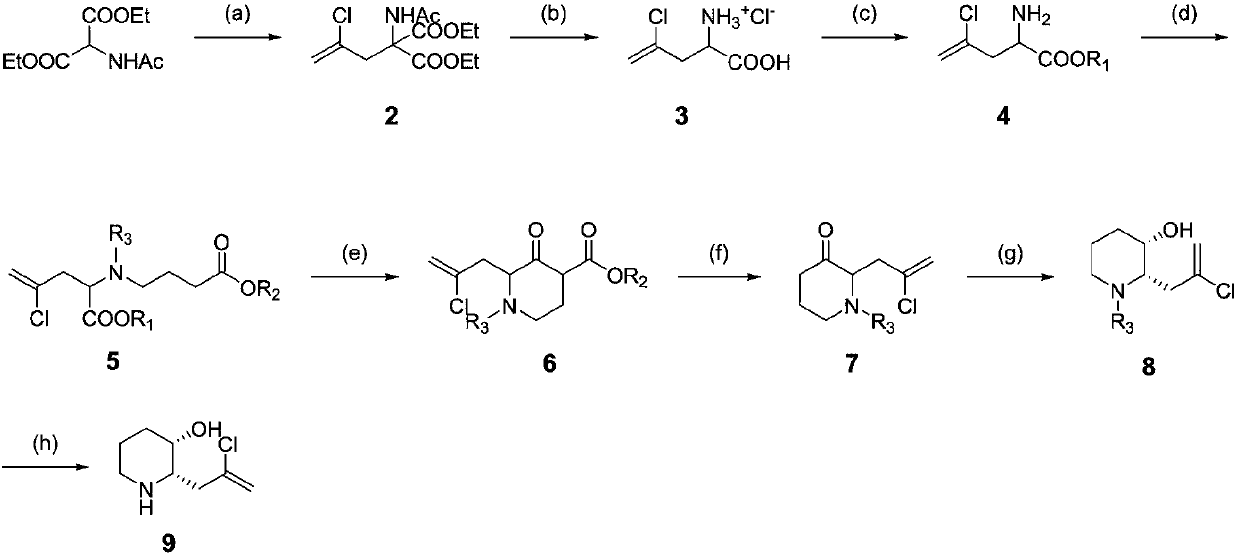
Synthetic method for halofuginone and intermediate of halofuginone
A synthesis method and a halofuginone technology are applied in the field of synthesis of halofuginone and its intermediates, and can solve the problems of expensive raw materials, inability to meet the market demand of halofuginone, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Intermediate compound 4 (R 1 = Synthesis of Et)
[0180]
[0181] Step (a): At room temperature, add diethyl acetamidomalonate (5.00kg, 23.02mol), anhydrous potassium carbonate (6.35kg, 46.04mol), potassium iodide (0.76kg, 4.6mol) into a 50L reactor , tetrabutylammonium bromide (0.37kg, 1.15mol) and acetonitrile (25L), after stirring for 20 minutes, 2,3-dichloropropene (3.07kg, 27.62mol) was added. The temperature was raised to 85-90°C for reaction, and the reaction was monitored by HPLC. After the reaction is finished, the temperature of the reaction liquid is lowered to within 25°C, and the diluted hydrochloric acid is slowly dropped into the reaction kettle to neutralize to pH 7-7.5. After standing still, the layers were separated, and the organic layer was concentrated under reduced pressure at 50°C. The concentrated residue was added with ethanol-water (1:10, 20 L) and stirred for 1 hour to crystallize. Filter under reduced pressure, rinse the filter cake wi...
Embodiment 2
[0243] Synthesis of intermediate compound 13
[0244]
[0245] Step (i): In the 50L reactor at room temperature, add compound 9 (0.7kg, 3.98mol), 1,4-dioxane (3.5L), water (3.5L), sodium carbonate (0.63kg, 5.98 mol), stirred and cooled to 5-10°C. 9-Fluorenylmethyl chloroformate (1.03kg, 3.98mol) was dissolved in 1,4-dioxane (1.4L) and added dropwise to the reaction kettle, and the temperature was controlled not to exceed 20°C. After dropping, the reaction was continued to stir at room temperature and monitored by TLC. After the reaction was completed, ethyl acetate (15 L) and water (15 L) were added and stirred for liquid separation. The organic layer was washed with saturated sodium chloride (3L×2), and the layers were separated. The aqueous layer was extracted once with ethyl acetate (8 L). Combine the organic layers, add activated carbon (300 g) and stir at room temperature for 1 hour. After filtration, the filtrate was concentrated under reduced pressure to obtain ...
Embodiment 3
[0256] Intermediate compound 4 (R 1 = Synthesis of Et)
[0257]
[0258] Step (a): At room temperature, add diethyl acetamidomalonate (2.50kg, 11.51mol), anhydrous potassium carbonate (3.18kg, 23.02mol), potassium iodide (0.38kg, 2.3mol) into a 50L reactor , tetrabutylammonium bromide (0.19kg, 0.58mol) and acetonitrile (13L), after stirring for 20 minutes, 2,3-dichloropropene (1.53kg, 13.81mol) was added. The temperature was raised to 85-90°C for reaction, and the reaction was monitored by HPLC. After the reaction is finished, the temperature of the reaction liquid is lowered to within 25°C, and the diluted hydrochloric acid is slowly dropped into the reaction kettle to neutralize to pH 7-7.5. After standing still, the layers were separated, and the organic layer was concentrated under reduced pressure at 50°C. The concentrated residue was added with ethanol-water (1:10, 10 L) and stirred for 1 hour to crystallize. Filter under reduced pressure, rinse the filter cake wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com