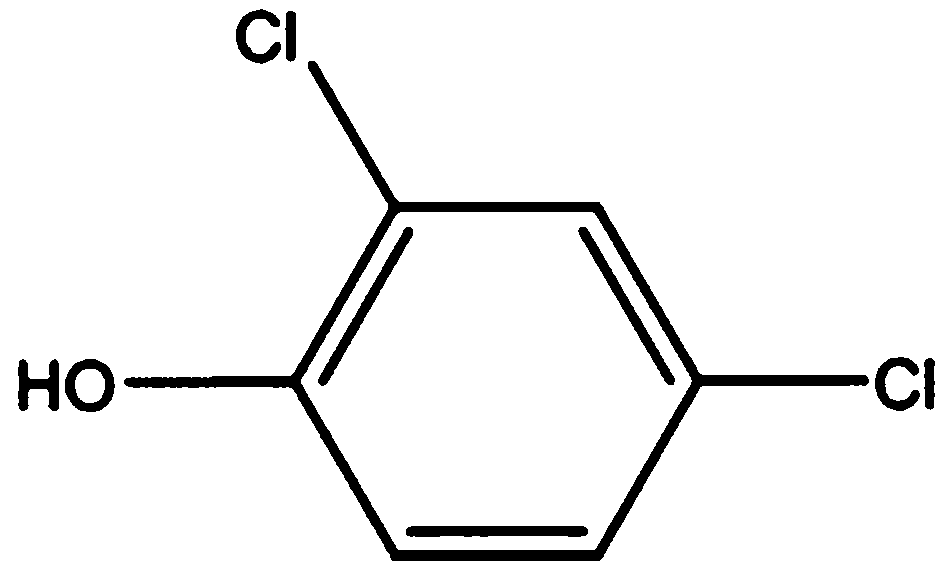
Method for preparing 2,4-dichlorophenol by catalytic chlorination of phenol
A phenol-catalyzed chlorine and dichlorophenol technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of complex processing, high cost, increased production cost, etc., and achieve high yield and low impurity content low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Serial number Retention time, min Component name 113.837 2-chlorophenol 217.778 phenol 321.724 2,4-Dichlorophenol 435.387 4-chlorophenol
[0025] *The retention time of each component will have a small deviation every time the gas chromatographic detection is performed
[0026] The chlorinated phenol product was diluted with toluene and then tested in the gas phase. Under the same conditions, pure toluene, 2-chlorophenol, phenol, 2,4-dichlorophenol, and 4-chlorophenol were tested in the gas phase. The retention time of each substance was determined as shown in the table above. , The retention time is toluene at 4.0min, which is not considered here.
[0027] After the reaction, the GC test results are as follows figure 1 As shown, the dilution solvent toluene with a retention time of about 4.0 is not discussed here. According to the previous description, the retention time at 21.733 min is 2,4-dichlorophenol. According to the area normalization method, the content of 2,4-...
Embodiment 2
[0030] Except that the co-catalyst was changed to 0.120g o-aminothiophenol, the rest was the same as in Example 1. The gas phase detection reaction mixture, the content of 2,4,-dichlorophenol was 97.2%.
Embodiment 3
[0032] Except that the co-catalyst was changed to 0.191g of phenothiazine, the rest was the same as in Example 1; the gas phase detection reaction mixture, the content of 2,4,-dichlorophenol was 97.8%.
[0033] Examples 1-3 tested the influence of the method by changing the promoter. It can be seen from the results that when Fe powder is used as the catalyst, the effect of using phenothiazine as the promoter is better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com