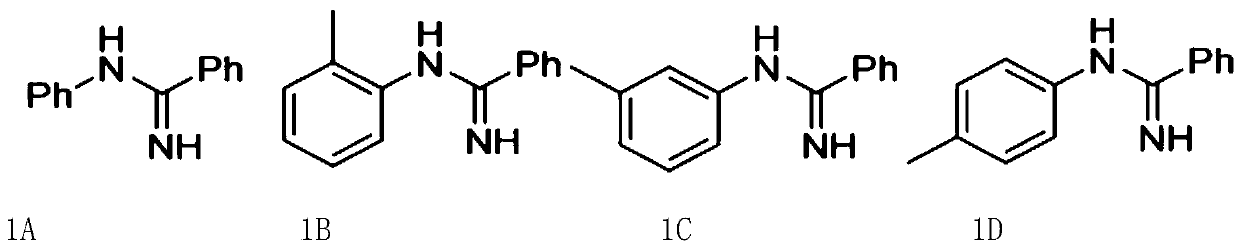
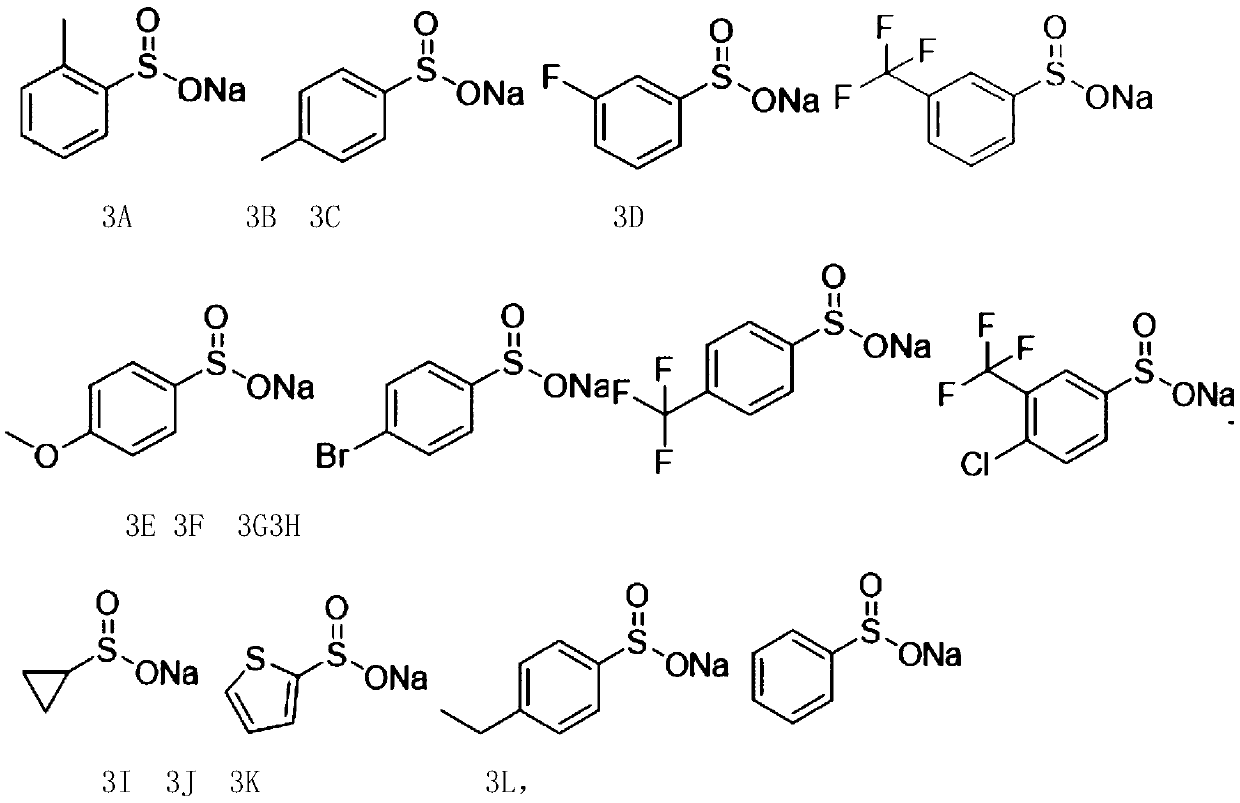
Arylamidine compounds and synthetic method thereof
A synthesis method and compound technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as insufficient abundance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Follow the steps below to synthesize aryl amidines:
[0178] 1) In a 50mL round-bottomed flask, mix 20mmoL of aniline and benzonitrile at a ratio of 1:1 and stir for 10 minutes, then add 1 equivalent of aluminum chloride in small amounts, heat to 200°C, and after 30 minutes Add 0.6mL of concentrated hydrochloric acid, 50mL of water, and 0.3g of activated carbon, continue stirring for 30 minutes, then stop stirring, cool to room temperature and filter, pour the filtered filtrate into a 100mL beaker containing 6.6g of sodium hydroxide and 36mL of water to produce floc, the floc is suction filtered, washed with water, and dried at room temperature to constant weight to obtain the aryl amidine;
[0179] 2) Put 0.2mmoL of compound 1, 1 equivalent of phenylpropynaldehyde, 1 equivalent of sodium benzenesulfinate, 1 equivalent of acetic acid, and 2 mL of ethanol in a 25 mL test tube, and react for 4 hours under a heating mantle at 70°C The reaction solution was obtained, extra...
Embodiment 2
[0342] Follow the steps below to synthesize aryl amidines:
[0343] 1) In a 50mL round bottom flask, mix aniline and benzonitrile for 8 minutes, then add aluminum trichloride several times in small amounts, heat to 180°C, add concentrated hydrochloric acid, water, and activated carbon after 20 minutes, and continue stirring Stop stirring after 20 minutes, filter after cooling to room temperature, pour the filtrate obtained by filtration into a 100mL beaker containing sodium hydroxide and water to produce flocs, wash the flocs by suction filtration, and dry at room temperature to constant weight to obtain The mass ratio of arylamidine, aniline, benzonitrile, aluminum trichloride, concentrated hydrochloric acid, water, activated carbon, and sodium hydroxide solution is 1:1.1:1.42:0.37:26.83:0.15:22.86, and the mass ratio of sodium hydroxide solution The concentration is 15.5%;
[0344] 2) Put compound 1, phenylpropynaldehyde, sodium benzenesulfinate, acetic acid, and ethanol in...
Embodiment 3
[0350] Follow the steps below to synthesize aryl amidines:
[0351] 1) In a 50mL round bottom flask, mix aniline and benzonitrile for 12 minutes, then add aluminum trichloride several times in small amounts, heat to 220°C, add concentrated hydrochloric acid, water, and activated carbon after 40 minutes, and continue stirring Stop stirring after 40 minutes, filter after cooling to room temperature, pour the filtrate obtained by filtration into a 100mL beaker containing sodium hydroxide and water to produce flocs, wash the flocs by suction filtration, and dry to constant weight at room temperature to obtain The mass ratio of aryl amidine, aniline, benzonitrile, aluminum trichloride, concentrated hydrochloric acid, water, activated carbon, sodium hydroxide solution is 1:1.12:1.44:0.39:26.85:0.17:22.88, the mass ratio of sodium hydroxide solution The concentration is 15.5%;
[0352] 2) Put compound 1, phenylpropynaldehyde, sodium benzenesulfinate, acetic acid, and ethanol in a 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com