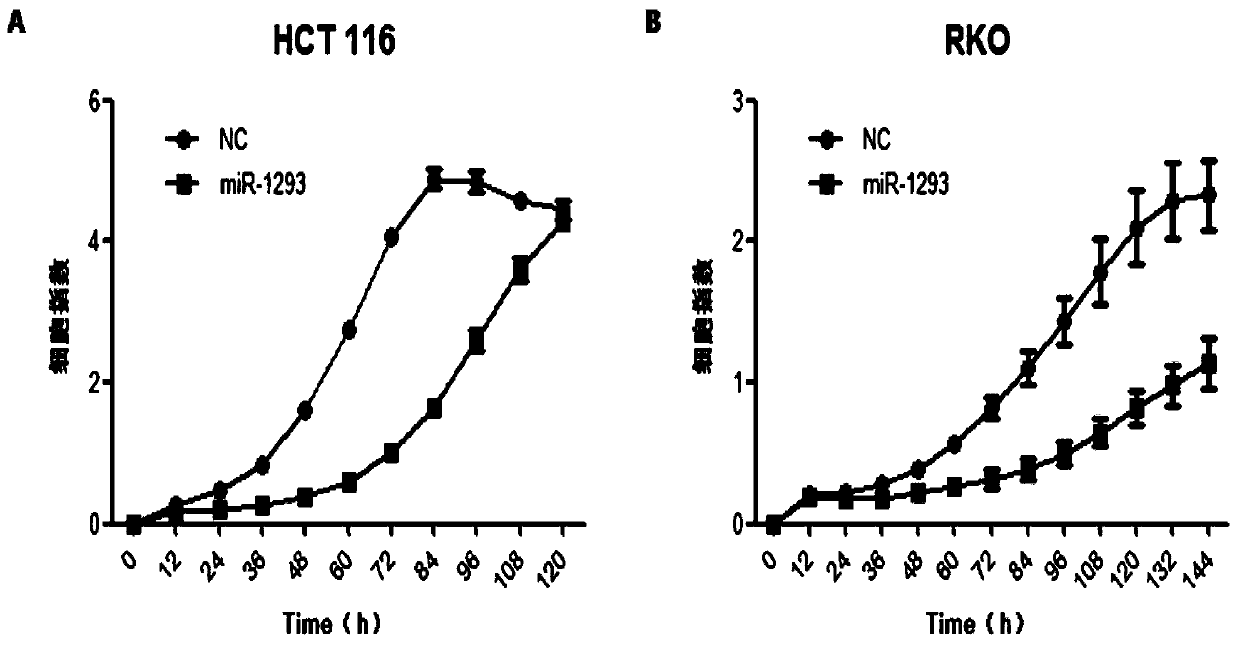
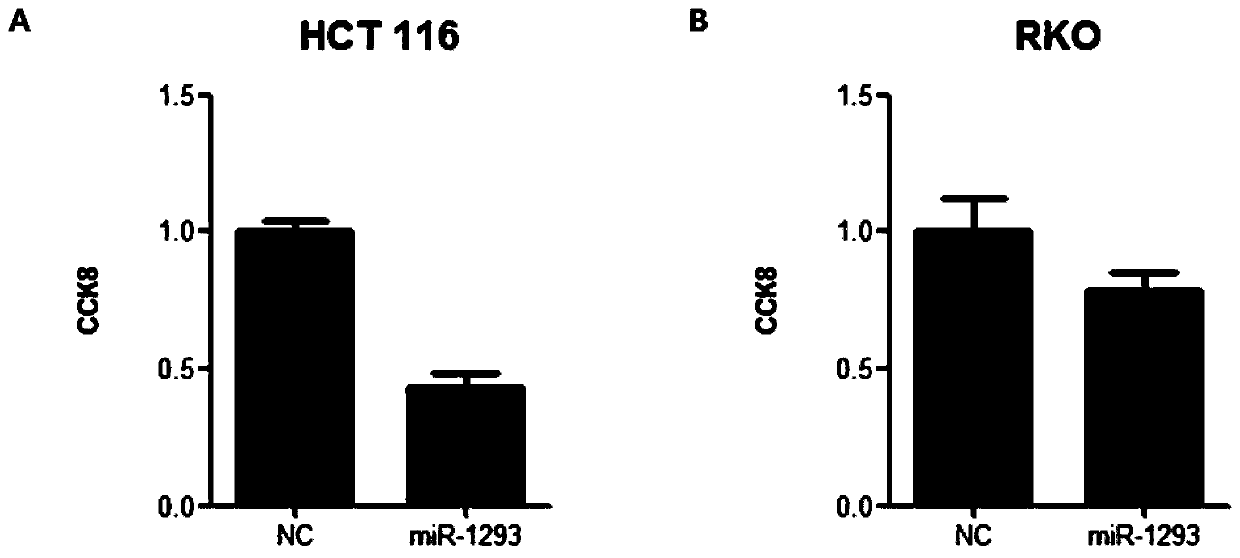
Application of miRNA-1293 in preparation of anti-colorectal tumor drug
The technology of mirna-1293 and 1. mirna-1293 is applied in the application field of preparing anti-colorectal tumor drugs, and can solve the problems of insensitivity to chemotherapy drugs, harmful and unhelpful, tumor recurrence, etc., and achieve the effect of high-efficiency anti-tumor effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 colorectal tumor cell culture
[0040] Human colorectal tumor cells HCT 116 and RKO (purchased from ATCC, the American Type Culture Collection) were cultured in RPMI-1640 and DMEM medium containing 10% FBS at 37°C, 5% CO 2 , saturated humidity in a carbon dioxide incubator. When the cell confluence reached 80%-90%, the cells were digested and passaged with 0.25% trypsin.
Embodiment 2
[0041] Example 2 Cell Transfection
[0042] 1. use RNAiMAX transfection reagent transfected HCT 116 and RKO cells with miRNA-1293mimics and negative control NC mimics respectively:
[0043] The specific sequences of miRNA-1293mimics and negative control NC mimics (purchased from Gemma Gene Company) are as follows:
[0044] NC mimics:
[0045] SEQ ID NO.1 5'-UUCUCCGAACGUGUCACGUTT-3'
[0046] SEQ ID NO.2 5'-ACGUGACACGUUCGGAGAATT-3'
[0047] miR-1293mimics:
[0048] SEQ ID NO.3 5'-UGGGUGGUCUGGAGAUUUGUGC-3'
[0049] SEQ ID NO.4 5'-ACAAAUCUCCAGACCACCAUU-3'
[0050] 2. The specific experimental steps are as follows:
[0051] (1) Digest well-growing HCT 116 and RKO cells in the logarithmic growth phase with trypsin, and press HCT 116 cells per well at 3×10 5 3×10 RKO cells per well 5 The cell density of each was planted in a 6-well plate, cultured in RPMI-1640 and DMEM medium, and placed in a carbon dioxide incubator for 24 hours;
[0052] (2) After culturing overnight, di...
Embodiment 3
[0055] Example 3 Q-PCR detection of the expression level of miRNA-1293
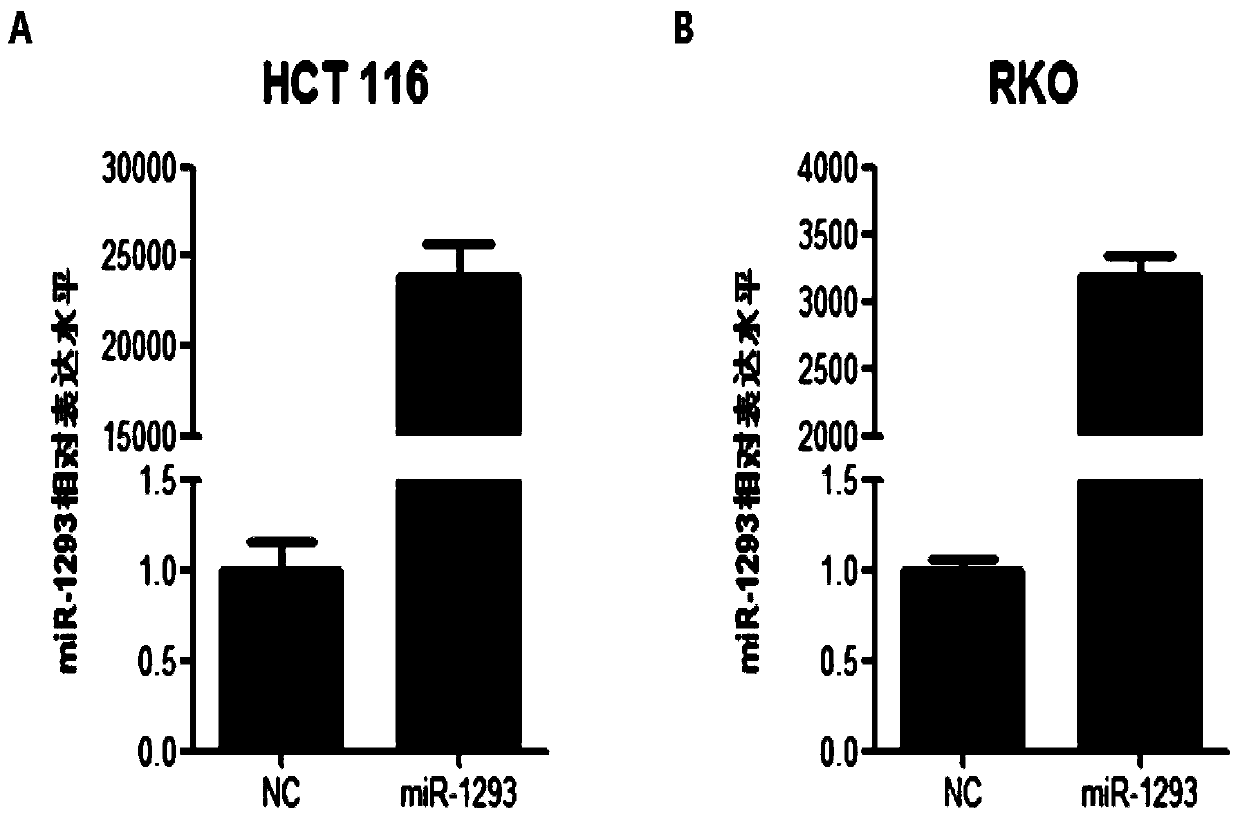
[0056] After transfecting HCT 116 cells and RKO cells (cells obtained in step (4) in Example 2) with NC mimics and miRNA-1293mimics, RNA was extracted, reversed by tailing, and its expression level was detected by Q-PCR. Specific steps are as follows:
[0057] 1. Collect HCT 116 and RKO cells 48 hours after transfection with Trizol reagent, and extract RNA:
[0058] (1) Suck off the medium in the well plate, wash 2-3 times with PBS, remove the residual medium as much as possible, then add 1mL Trizol reagent to each well to lyse the cells, pipette continuously until clear, and then The lysate was transferred into a 1.5mL centrifuge tube and left at room temperature for 5 minutes;
[0059] (2) Add 200 μL of chloroform to each tube, mix well, and let stand at room temperature for 3 minutes;
[0060] (3) Centrifuge at 4°C and 12000rpm for 10min, and transfer the upper aqueous phase to another new 1.5mL cen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com