Preparation method for improving purity of terbinafine hydrochloride
A technology for terbinafine hydrochloride and purity, which is applied in the field of preparation for improving the purity of terbinafine hydrochloride, can solve the problems of difficult product purification and high production cost, and achieve the effects of improving purity, low cost, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
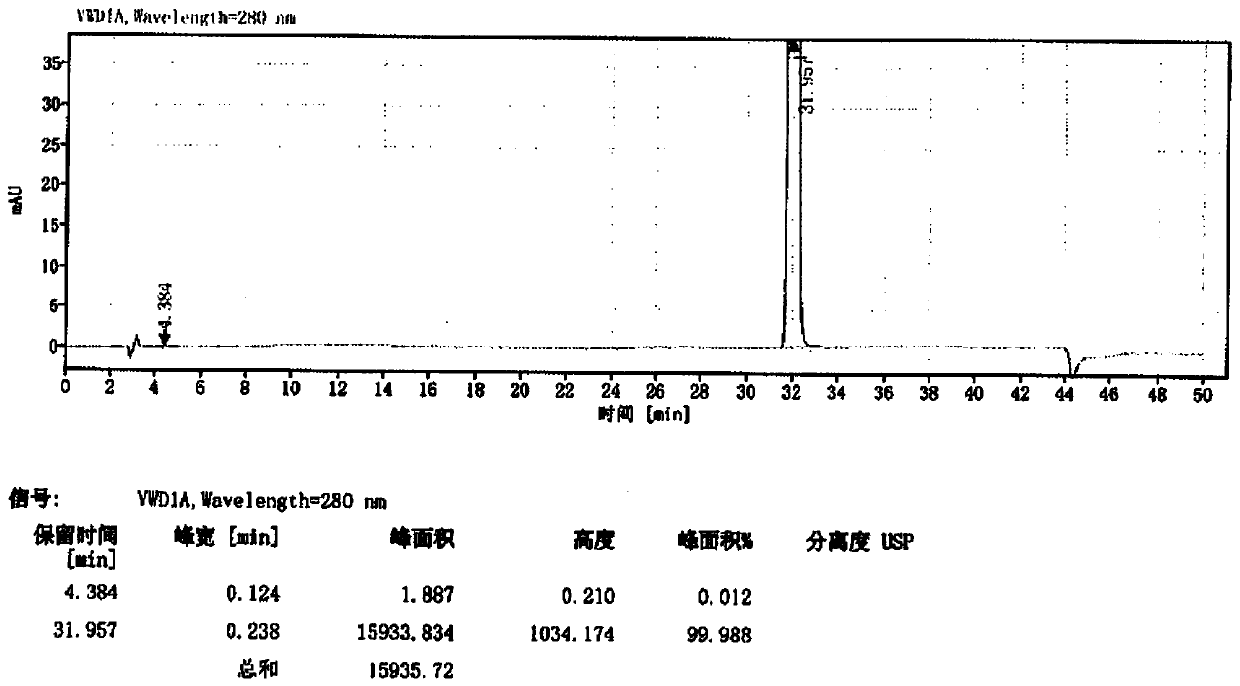
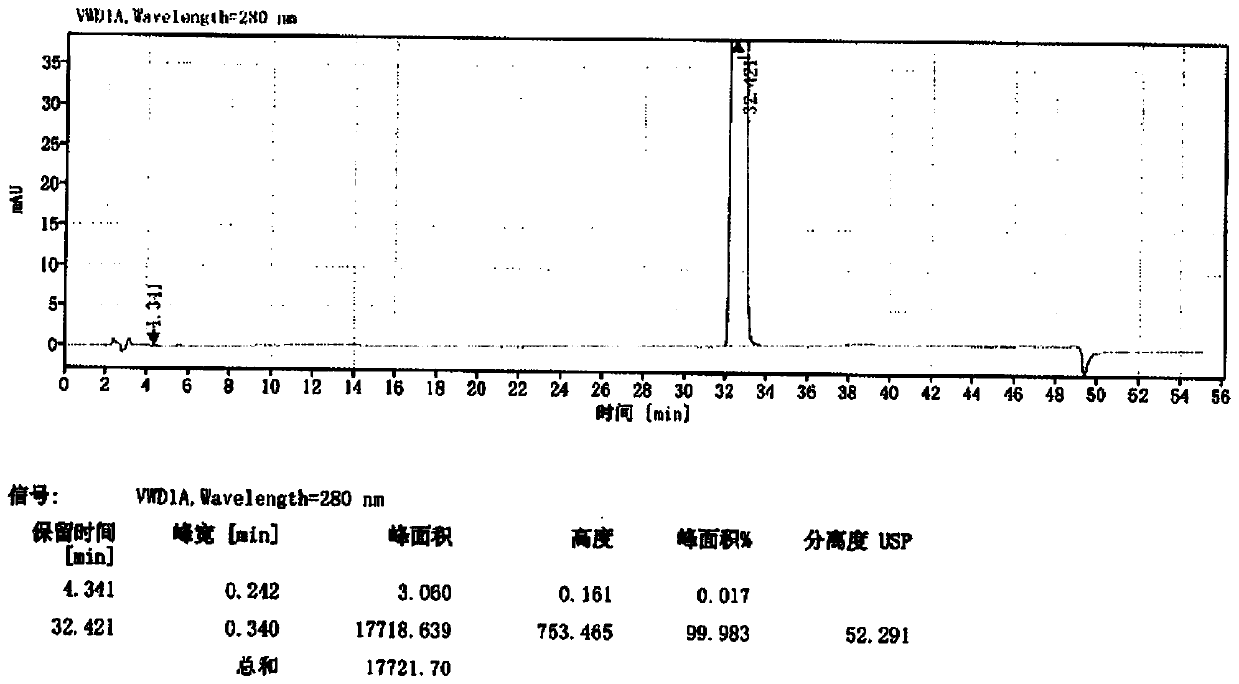
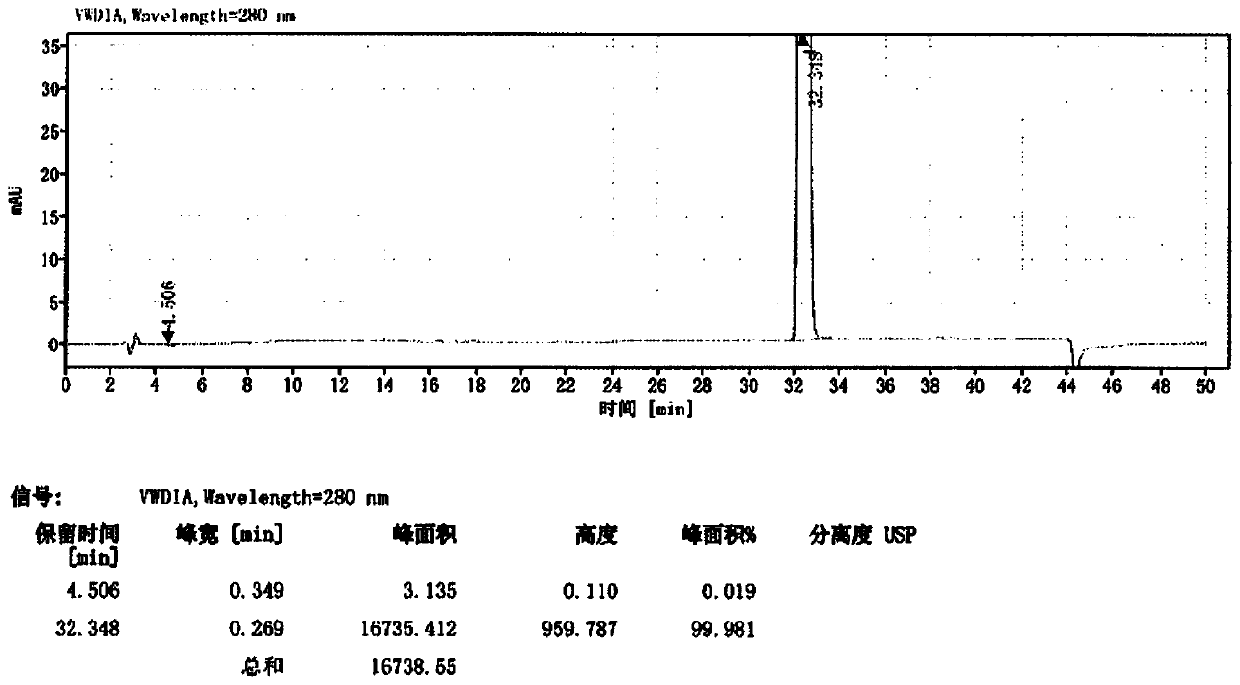
Image
Examples
Embodiment 1
[0024] Step 1 (synthesis of terbinafine hydrochloride crude product):
[0025] Add 12.5ml of purified water and potassium carbonate (4.06g, 29.2mmol) to a 100ml three-necked reaction flask, stir and dissolve, then add N-methylnaphthylmethylamine (5.01g, 29.2mmol), then add 1-chloro-6,6 -Dimethyl-2-hepten-4-yne (4.57g, 29.2mmol), heated to 80°C for reaction, after 4 hours, cooled to room temperature, added 25ml ethyl acetate for extraction, separated the ethyl acetate phase and cooled To 0-10°C, add 6N hydrochloric acid aqueous solution dropwise, adjust the pH to 1, stir for 1-2h, filter, and dry the filter cake in vacuum at 70°C for 4h to obtain crude terbinafine hydrochloride (8.51g, yield: 89% );
[0026] Step 2 (purification of terbinafine hydrochloride crude product):
[0027] Add crude terbinafine hydrochloride (8.51g, 26.8mmol) and 106ml of purified water to a 250ml single-necked bottle, heat to reflux until dissolved, cool to room temperature naturally, cool to 0-10°C...
Embodiment 2
[0029] Step 1 (synthesis of terbinafine hydrochloride crude product):
[0030] Add 625ml of purified water and potassium carbonate (203.21g, 1.46mol) to a 3L three-necked reaction flask, stir and dissolve, then add N-methylnaphthylmethylamine (250.02g, 1.46mol), then add 1-chloro-6,6- Dimethyl-2-hepten-4-yne (228.07g, 1.46mol), heated to 80°C for reaction, after 4 hours, cooled to room temperature, added 1.25L ethyl acetate for extraction, separated the ethyl acetate phase and cooled to 0-10°C, add 6N hydrochloric acid aqueous solution dropwise, adjust the pH to 1, stir for 1-2h, filter, and vacuum-dry the filter cake at 70°C for 4h to obtain crude terbinafine hydrochloride (435.04g, yield: 91%) ;
[0031] Step 2 (purification of terbinafine hydrochloride crude product):
[0032] Add crude terbinafine hydrochloride (435.04 g, 1.33 mol) and 5220 ml of purified water to a 10 L three-necked flask, heat to reflux until dissolved, cool to room temperature naturally, cool to 0-10 ...
Embodiment 3
[0034] Step 1 (synthesis of terbinafine hydrochloride crude product):
[0035] Add 62.62L of purified water and potassium carbonate (20.36Kg, 146.49mol) to the 200L reactor, stir and dissolve, then add N-methylnaphthylmethylamine (25.05Kg, 146.49mol), then add 1-chloro-6,6- Dimethyl-2-hepten-4-yne (22.85Kg, 146.49mol), heat up to 80°C for reaction, after 4 hours, cool to room temperature, add 125L ethyl acetate for extraction, separate the ethyl acetate phase and cool to 0 -10°C, add 6N hydrochloric acid aqueous solution dropwise, adjust the pH to 1, stir for 1-2h, filter, and vacuum-dry the filter cake at 70°C for 4h to obtain crude terbinafine hydrochloride (44.07Kg, yield: 92%);
[0036] Step 2 (purification of terbinafine hydrochloride crude product):
[0037] Add the crude product of terbinafine hydrochloride (44.07Kg, 134.77mol) and 528.84L of purified water into the 800L reaction kettle, heat and reflux until it dissolves, cool to room temperature naturally, cool to 0-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com