Toddacoumalone compound or pharmaceutically acceptable salt and preparation method and application thereof
A compound and pharmaceutical technology, applied in the fields of organic chemistry, organic chemistry, pharmaceutical formulations, etc., can solve problems such as synthesis that have not been reported in the literature, and achieve the effects of high yield and short reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
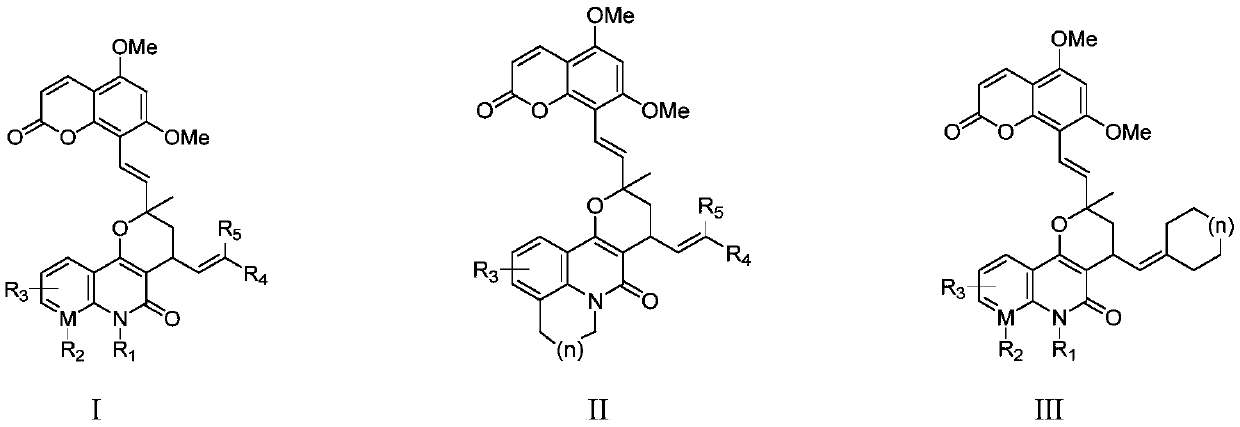
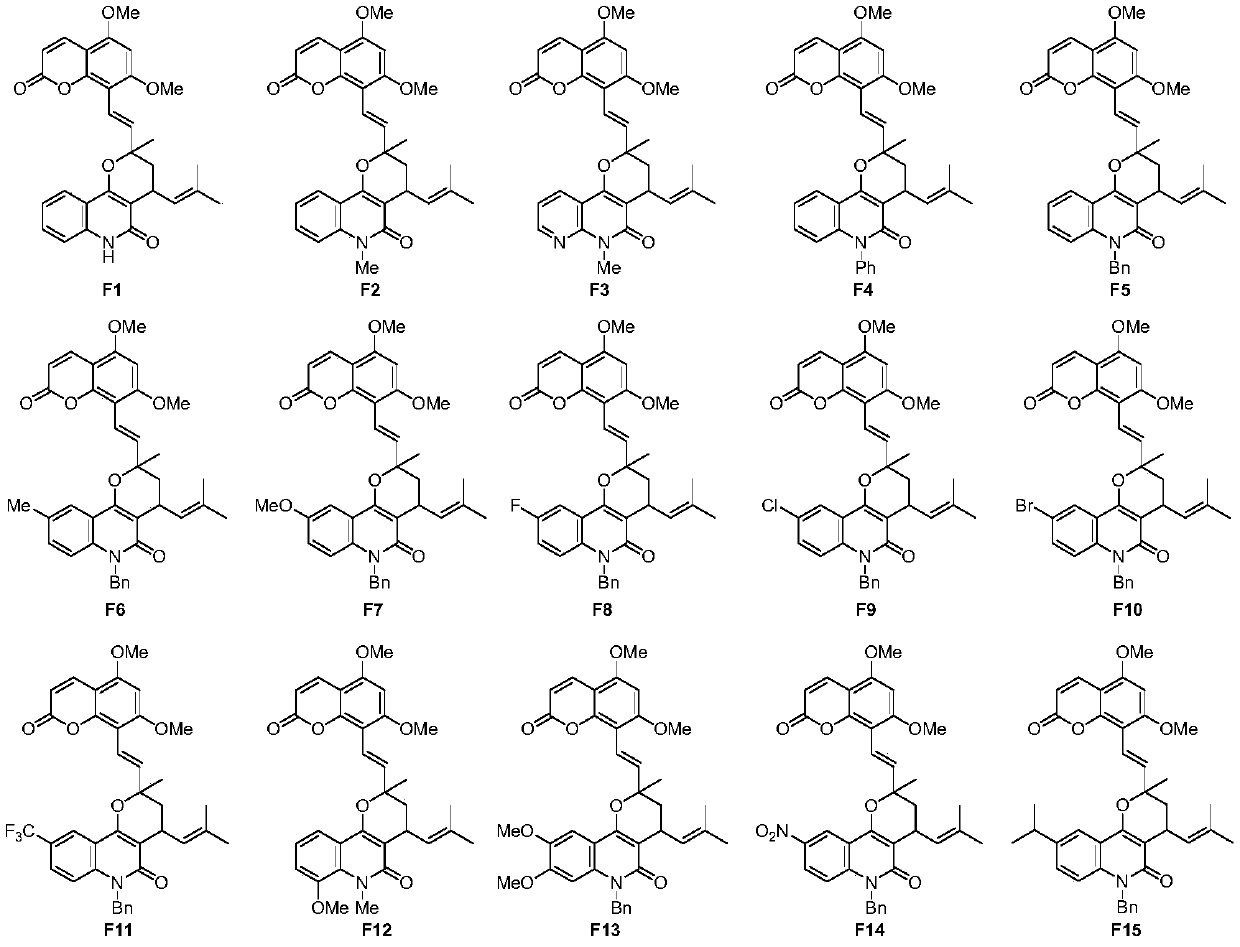
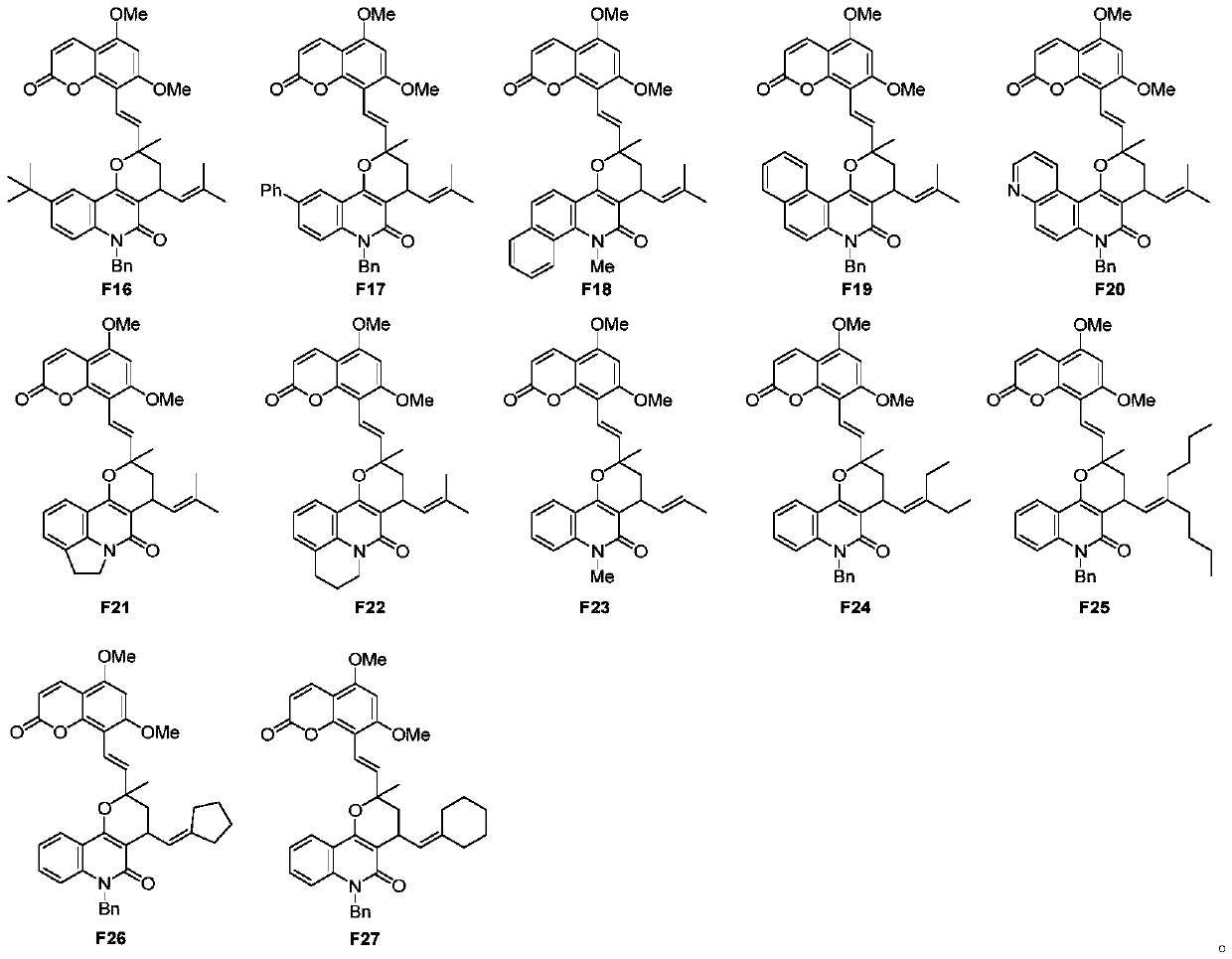
Image
Examples
Embodiment 1
[0065] The preparation of embodiment 1 compound F1
[0066] 1, the preparation of intermediate product (B1): with quinolinone A1 (5mmol, 1eq) and aldehyde G1 (6mmol, 1.2eq) as substrate (the specific structure of substrate is shown in Table 1), with pyridine as base and solvent ( 10 mL), refluxed at 100°C for 3 hours, cooled, concentrated, then washed with dilute hydrochloric acid to remove residual pyridine, then washed the organic phase twice with saturated brine, combined the organic phases, dried over anhydrous sodium sulfate, concentrated, and The residue was separated by column chromatography to obtain the intermediate product (B1) with a yield of 79%. The characterization data of above-mentioned intermediate product (B1) is:
[0067] 1 H NMR (400MHz, CDCl 3 )δ=10.76(s,1H),7.89(dd,J=8.0,0.8Hz,1H),7.50-7.44(m,1H),7.28(s,1H),7.23–7.16(m,1H),6.75 (d,J=10.0Hz,1H),5.55(d,J=10.0Hz,1H),1.63(s,6H). 13 C NMR (100MHz, CDCl 3 ) δ = 162.80, 157.23, 138.02, 130.77, 126.15, 122....
Embodiment 2
[0075] The preparation of embodiment 2 compound F2
[0076] 1, the preparation of intermediate product (B2)
[0077] It was prepared according to the method for preparing intermediate product B1 in Example 1, except that the substrates were quinolinone A2 and aldehyde G1 (see Table 1 for the specific structure of the substrate), and the yield was 87%. Its characterization data are:
[0078] 1 H NMR (400MHz, CDCl 3)δ=7.97(d, J=8.0Hz, 1H), 7.59–7.51(m, 1H), 7.32(d, J=8.4Hz, 1H), 7.23(t, J=7.6Hz, 1H), 6.76( d,J=10.0Hz,1H),5.54(d,J=10.0Hz,1H),3.70(s,3H),1.52(s,6H). 13 C NMR (100MHz, CDCl 3 )δ=161.01, 155.18, 139.37, 130.84, 126.33, 123.13, 121.69, 117.99, 116.11, 114.00, 105.88, 78.74, 29.26, 28.23.
[0079] 2. Preparation of intermediate product (D2)
[0080] According to the method for preparing intermediate product D1 in Example 1, the yield was 65%. Its characterization data are:
[0081] 1 H NMR (400MHz, CDCl 3 )δ=7.91(dd,J=8.0,1.2Hz,1H),7.57-7.52(m,1H),7.33(d,J=8....
Embodiment 3
[0089] The preparation of embodiment 3 compound F3
[0090] 1, the preparation of intermediate product (B3):
[0091] It was prepared according to the method for preparing intermediate product B1 in Example 1, except that the substrates were quinolinone A3 and aldehyde G1 (see Table 1 for the specific structure of the substrate), and the yield was 75%. Its characterization data are:
[0092] 1 H NMR (400MHz, CDCl 3 )δ=7.97(d, J=8.0Hz, 1H), 7.59–7.51(m, 1H), 7.23(t, J=7.6Hz, 1H), 6.76(d, J=10.0Hz, 1H), 5.54( d,J=10.0Hz,1H),3.70(s,3H),1.52(s,6H). 13 CNMR (100MHz, CDCl 3 )δ=161.01, 155.18, 130.84, 126.33, 123.13, 121.69, 117.99, 116.11, 114.00, 105.88, 78.74, 29.26, 28.23.
[0093] 2. Preparation of intermediate product (D3):
[0094] According to the method for preparing intermediate product D1 in Example 1, the yield was 62%. Its characterization data are:
[0095] 1 H NMR (400MHz, CDCl 3 )δ=7.91(dd,J=8.0,1.2Hz,1H),7.57-7.52(m,1H),7.26–7.19(m,1H),5.09–5.01(m,1H),3.98...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com