1-(3,5-dimethoxy phenyl)-3-substituted urea colon cancer inhibitor as well as preparation and application thereof
A kind of technology of dimethoxyphenyl and substituted urea, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 compound
[0045] 1.1 The specific synthetic route of the compound is as follows:
[0046]
[0047] 1.2 Synthesis steps
[0048] a. the synthesis of the first step intermediate product:
[0049] Step 1: Take a dry three-neck reaction bottle and put it into a magnet. Add 4-amino-6-chloropyrimidine (1eq), KI (0.5eq), and dissolve with absolute ethanol (35mL). After heating with stirring for 10 min on a magnetic stirrer, trifluoroacetic acid (200 mL) was added. activation. After about 1 h, substituted benzylamine (0.8 eq) dissolved in absolute ethanol (15 mL) was added for reaction. Note that it should be added in a dropwise manner to achieve the effect of long-term excess reaction, and the dropping time should be controlled at about 1 hour.
[0050] Step 2: use TLC method to detect the reaction progress and reaction effect. Generally, after 36 hours of reaction, the reaction is almost complete. After the reaction is complete, first...
Embodiment 2
[0111] Example 2 compound anti-tumor cell activity
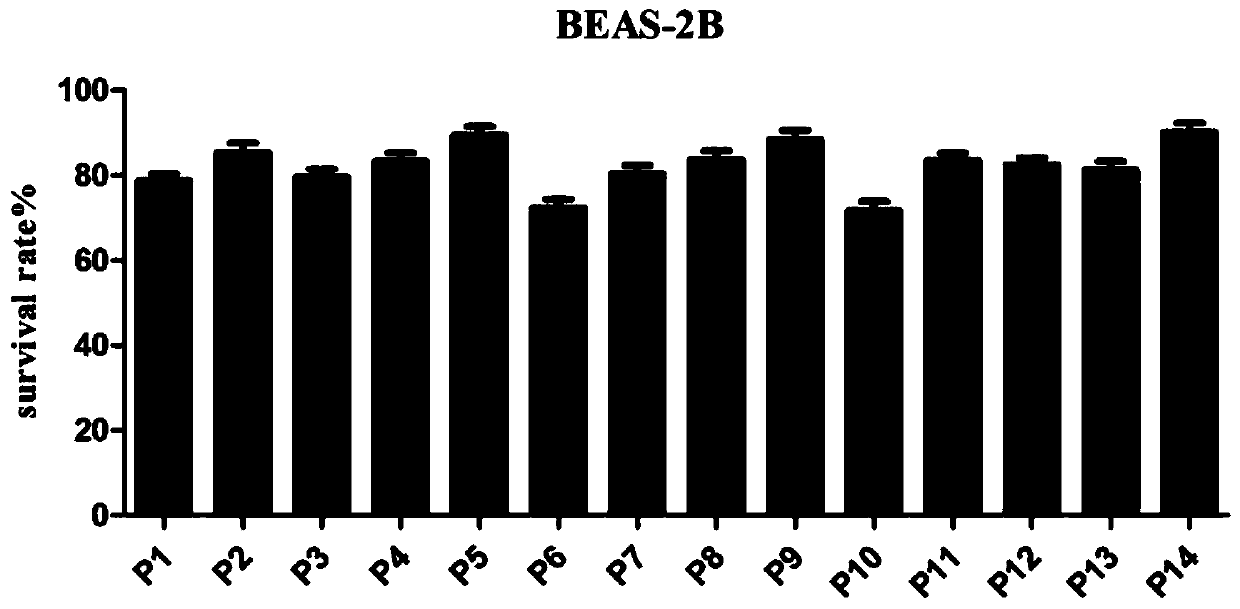
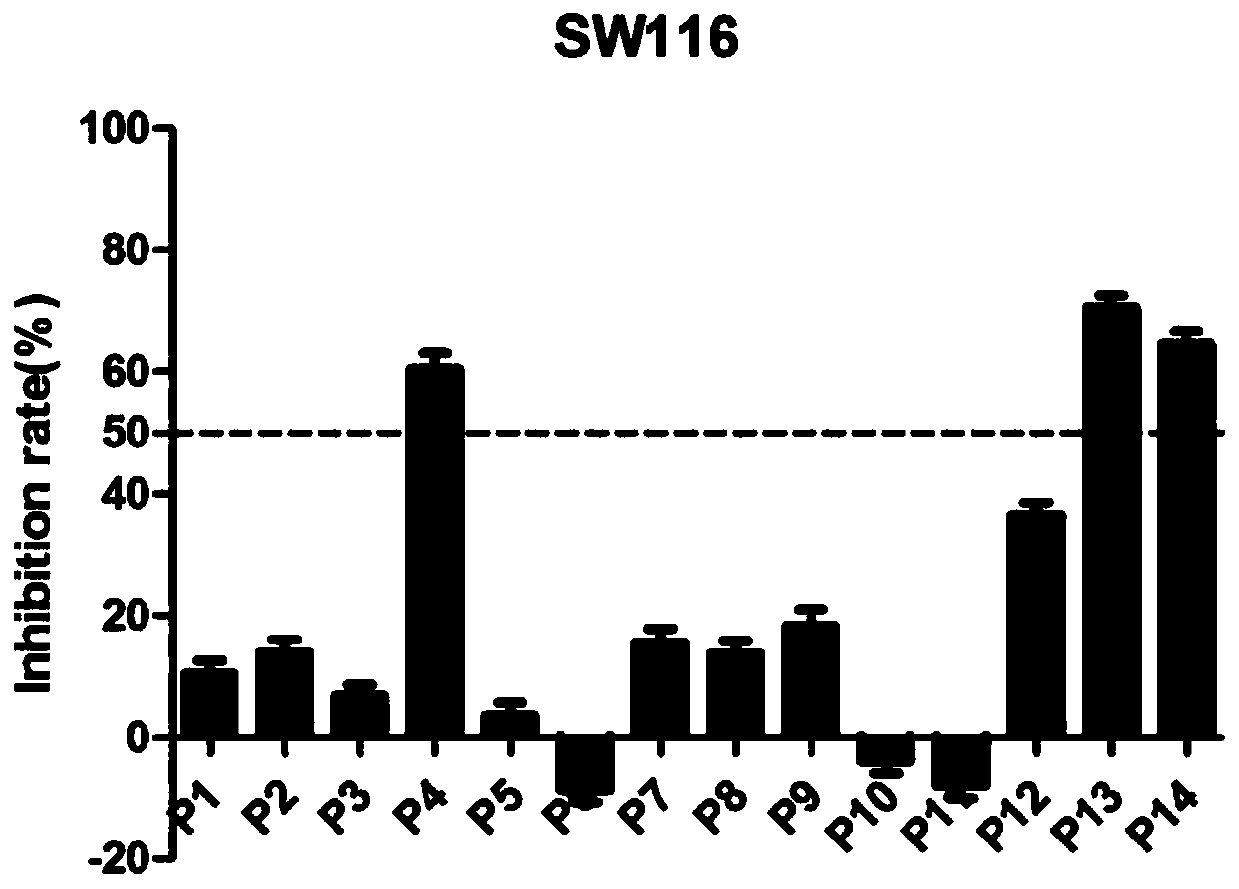
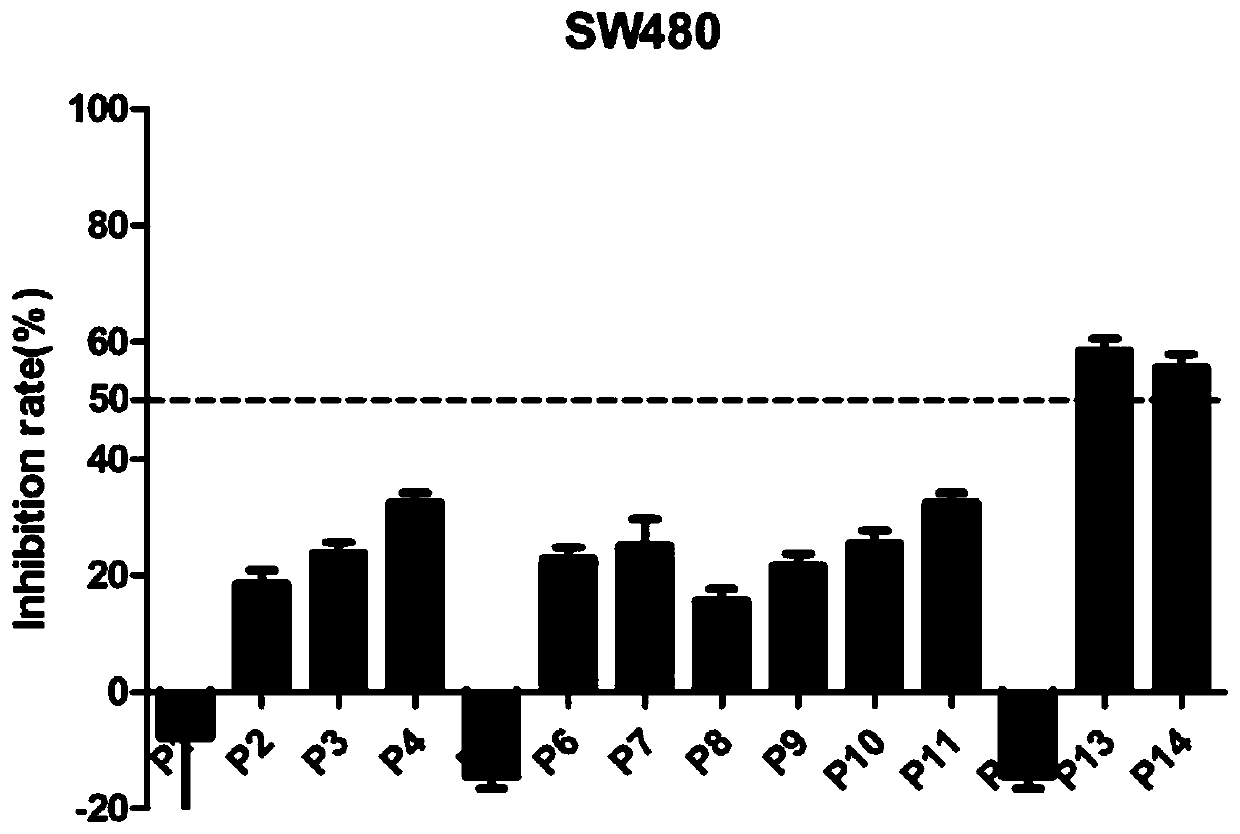
[0112] 2.1 MTT method to test the antitumor activity of compounds
[0113] This experiment uses the MTT method. The selected normal lung cells are BEAS-2B cells; the selected three cancer cells include SW116 cells (human colorectal cancer cells), SW480 cells (human colon cancer cells) and SW620 cells (human colon cancer cells). The above-mentioned cells with logarithmic growth were selected, digested, collected, and counted with a cell counting plate. Next, dilute the counted cells to an appropriate concentration (5*10^4 cells / mL~8*10^4 cells / mL), and add the diluted cell suspension to the 96-well plate at 100 μL per well culture medium, and remember to set blank control wells containing only medium on the same well plate; after overnight culture on the plate, replace with fresh medium, and add a series of concentration gradient dilutions of the test target compound to each well to wait for the drug effect After 72 hours,...
Embodiment 3
[0116] IC of Example 3 Compounds P13 and P14 to Three Kinds of Tumor Cells 50 experiment
[0117] 3.1 MTT method to test the IC of the compound 50 value
[0118] According to the results of the toxic effects of all target compounds on normal lung cells and the inhibitory effects on three colon cancer cell lines, we obtained compounds P13 and P14 that have inhibitory effects on all three colon cancer cell lines. We choose these two compounds, further to do IC 50 experiment. Six concentrations (20 μM, 10 μM, 1.0 μM, 0.50 μM, 0.10 μM and 0.01 μM) were first set for both compounds. Then, the three colon cancer cell lines were treated with different concentrations of these two compounds. Obtain the optical density (OD) value, calculate the inhibition rate, and calculate the IC of different compounds by GraphPad Prism5 software 50 value.
[0119] 3.2 The experimental results are shown in the table below:
[0120]IC of compounds acting on SW116, SW480, SW620 cell lines 50
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com