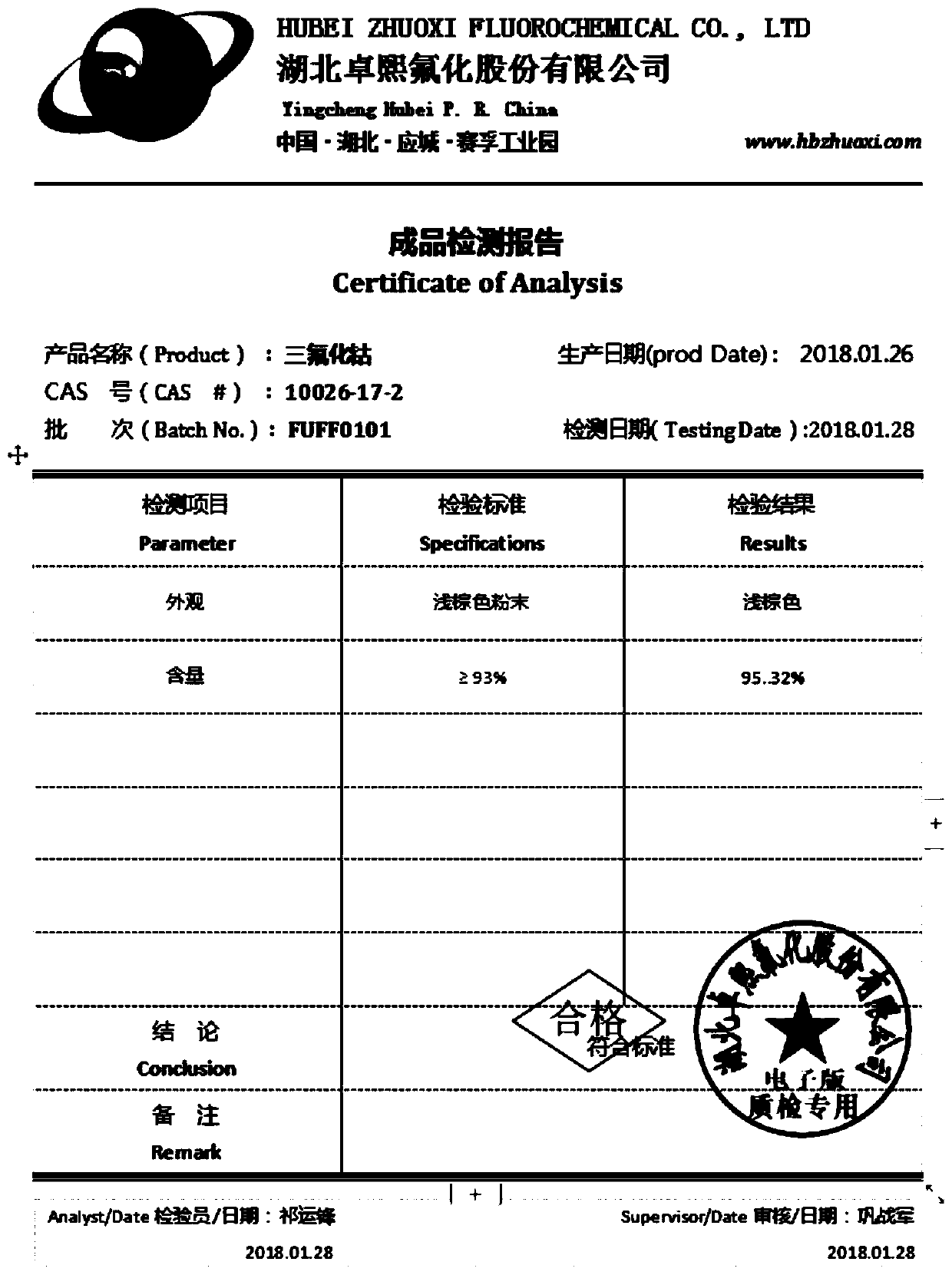
Preparation method of cobalt trifluoride
A technology of cobalt trifluoride and cobalt difluoride, applied in cobalt halide and other directions, can solve problems such as unclear structure, achieve good fluorination effect, ensure internal and external reactions, and reduce fluorine gas consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method for cobalt trifluoride, said method comprising the steps of:
[0024] Step 1, pulverize 2000g coke, mix with the ammonium chloride of 50g;
[0025] Step 2, transfer to the reactor for fluorination production. The reaction kettle has a heating device, and a drying device is installed at the outlet of the tail gas of the reaction kettle, and the tail gas is passed into an aqueous potassium hydroxide solution for absorption. After starting the heating device and raising the temperature to 50°C, start feeding fluorine gas until the pressure in the kettle is 2 kg, continue to raise the temperature to 200°C to start the reaction, and evacuate the pressure in the kettle after 20 minutes. Continue to feed fluorine gas to a pressure of 2 kg to continue the reaction. During the whole reaction process, the temperature in the kettle was controlled at about 200°C.
[0026] Until the completion of the reaction, 1200 g of fluorine gas was consumed. A total of ...
Embodiment 2
[0028] A preparation method for cobalt trifluoride, said method comprising the steps of:
[0029] Step 1, pulverize 2100g of coke and mix with 35g of ammonium chloride;
[0030] Step 2, transfer to the reactor for fluorination production. The reaction kettle has a heating device, and a drying device is installed at the outlet of the tail gas of the reaction kettle, and the tail gas is passed into an aqueous potassium hydroxide solution for absorption. After starting the heating device and raising the temperature to 50°C, start feeding fluorine gas until the pressure in the kettle is 1.8 kg, continue to raise the temperature to 190°C to start the reaction, and evacuate the pressure in the kettle after 20 minutes. Continue to feed fluorine gas to a pressure of 1.8 kg to continue the reaction. During the whole reaction process, the temperature in the kettle was controlled at about 190°C.
[0031] Until the completion of the reaction, 1280g of fluorine gas was consumed. A tota...
Embodiment 3
[0033] A preparation method for cobalt trifluoride, said method comprising the steps of:
[0034] Step 1, pulverize 2000g of coke and mix with 100g of ammonium chloride;
[0035] Step 2, transfer to the reactor for fluorination production. The reaction kettle has a heating device, and a drying device is installed at the outlet of the tail gas of the reaction kettle, and the tail gas is passed into an aqueous potassium hydroxide solution for absorption. After starting the heating device and raising the temperature to 50°C, start feeding fluorine gas until the pressure in the kettle is 2.2 kg, continue to heat up to 210°C to start the reaction, and evacuate the pressure in the kettle after 20 minutes. Continue to feed fluorine gas to a pressure of 2.2 kg to continue the reaction. During the whole reaction process, the temperature in the kettle was controlled at about 210°C.
[0036] Until the completion of the reaction, 1210 g of fluorine gas was consumed. A total of 2369 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com