An anti-candida albicans composition containing sanguine and its preparation method
A technology of blood streptococcin and Candida albicans, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, antibacterial drugs, etc. The difficulty of clinical treatment of candidiasis and other problems, to achieve the effect of degradable safety and non-toxicity, relieve dry mouth, and degradation products are safe and non-toxic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
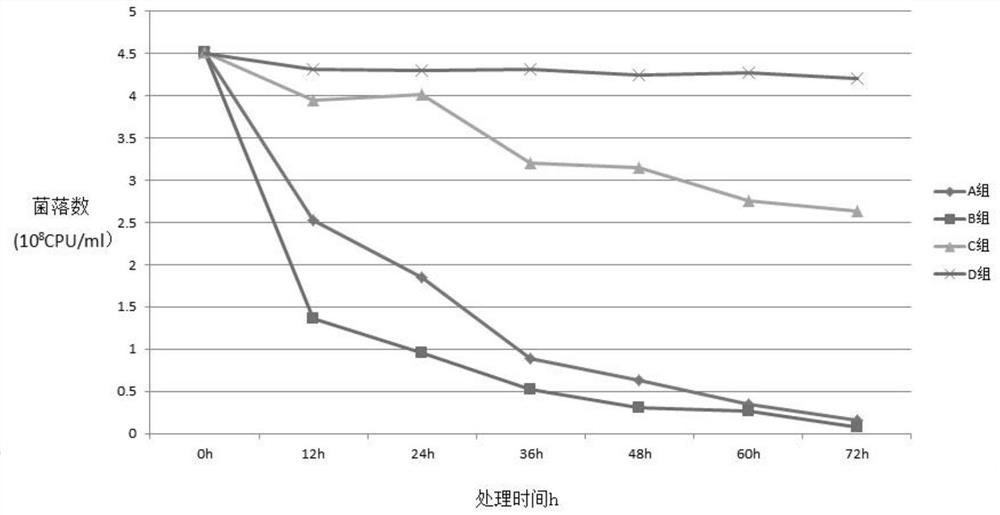
Image
Examples
Embodiment 1
[0033]An anti-Candida albicans composition containing streptococcin, which is a viscous gel dressing, in which the streptococcin is uniformly dispersed in the form of nanoscale liposomes, mainly including the following qualities Parts of components: 2-3 parts of streptococcin, 6-9 parts of PBS buffer solution, 10-1 parts of Tween 800.5 parts, 10-15 parts of polylactic acid-glycolic acid copolymer, 1-5 parts of collagen short peptide, vitamin 1-3 parts of B5, 1-3 parts of menthol, 1-2 parts of borneol, 2-5 parts of xylitol, 0.5-1 part of N-hydroxysuccinimide, 1-(3-dimethylaminopropyl)- 1-2 parts of 3-ethylcarbodiimide hydrochloride, 1.5-3 parts of dopamine and 100 parts of sodium hyaluronate aqueous solution with a mass concentration of 2-5%.
[0034] In this embodiment, streptococcin, PBS buffer solution, Tween 80 and polylactic acid-glycolic acid copolymer jointly form streptococcin liposome, and the drug loading rate of streptococcin in this liposome is 10~ 11%; Streptococc...
Embodiment 2
[0036] This embodiment provides an anti-Candida albicans composition containing streptococcin, which is a viscous gel dressing, and streptococcin is evenly dispersed in the viscous gel dressing in the form of nano-scale liposomes , mainly including the following components by mass: 3 parts of streptococcin, 9 parts of PBS buffer solution, 801 parts of Tween, 15 parts of polylactic acid-glycolic acid copolymer, 3 parts of collagen short peptide, 52 parts of vitamin B, menthol 2 parts, 1 part of borneol, 3 parts of xylitol, 1 part of N-hydroxysuccinimide, 2 parts of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, dopamine 3 parts and mass concentration are 100 parts of 5% sodium hyaluronate aqueous solution.
[0037] In this embodiment, streptococcin, PBS buffer solution, Tween 80 and polylactic acid-glycolic acid copolymer jointly constitute streptococcin liposome, and the drug loading rate of streptococcin in the liposome is 10.7%. ; Streptococcus liposomes and s...
Embodiment 3
[0039] This embodiment provides a method for preparing an anti-Candida albicans composition containing sanguine, comprising the steps of:
[0040] Step 1, extract blood streptococcin:
[0041] Proliferate and culture the purified and identified Streptococcus sanguis, inoculate the Streptococcus sanguis in BHI medium, and culture it under anaerobic conditions at 37°C for 48 hours, then centrifuge and collect the culture solution at 4°C. Wash bacterial sediment;
[0042] Disperse the obtained bacteria in PBS buffer solution to make a bacterial suspension, perform ultrasonic cell disruption on the bacterial suspension in an ice-water bath, and collect the supernatant of the cell disruption liquid by centrifugation at 4°C;
[0043] Ammonium sulfate was added to the supernatant of the resulting cell disruption liquid to make the ammonium sulfate in the supernatant reach a saturation of 60%, and it was placed at 4°C for salting out, and the first salting out solution was centrifuge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com