Cycloneolignan-type lignan enantiomers and their preparation and application
A technology of enantiomers and lignans, applied in medical preparations containing active ingredients, separation/purification of carboxylic acid esters, drug combinations, etc., can solve problems that have not yet been patented or reported in literature, and achieve The effect of novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
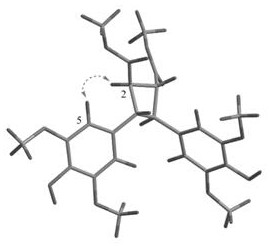
[0047] Embodiment 1: the preparation of formula 1a compound and formula 1b compound:
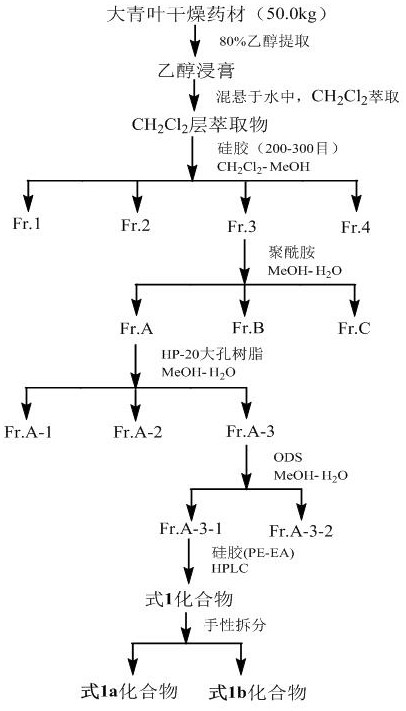
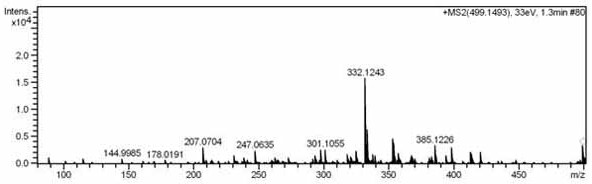
[0048] Take the dried Isatis indigo medicinal material, soak overnight with 2 times the volume of ethanol water, then extract 2-3 times with 5-8 times the volume of ethanol water, the volume concentration of ethanol is 70-80%, and the extraction adopts heating and reflux extraction or ultrasonic extraction. Combine all the extracts, concentrate under reduced pressure, recover the solvent, and dry to obtain the extract after completing the concentration under reduced pressure. The extract is separated by polyamide column chromatography, and the mixed solvent of ethanol-water with a volume ratio of 30-90% is used for gradient elution. Among them, 30% of the eluted part is separated by HP-20 macroporous resin column chromatography, and gradient elution is carried out with a mixed solvent of ethanol-water with a volume ratio of 0-90%. The 60% eluted part was separated by ODS column chromatogra...
Embodiment 2
[0049] Embodiment 2: MTT method detects the impact of formula 1a compound and formula 1b compound on human neuroblastoma SH-SY5Y cell viability:
[0050] 1. Cell culture
[0051] Neuroblastoma SH-SY5Y cell line (purchased from American Type Culture Collection ATCC, Manassas, USA) and DMEM medium containing 10% FBS (purchased from Logan Hyclone, Logan, USA), at 37°C, 5% CO 2 After 24 hours, the cells adhered to the wall, and the old culture medium was discarded.
[0052] 2. Cell grouping
[0053] Blank group: cultured only with DMEM complete medium without any drugs.
[0054] Model group: After the cells were cultured in DMEM complete medium for 4 hours, 1mM MPP was added + , continue to cultivate for 36 hours.
[0055] Group 1a: After the cells were cultured in complete DMEM medium for 4 hours, different concentrations (12.5 μM, 25 μM, 50 μM) of the compound of formula 1a were added for 1 hour, and then 1 mM MPP was added + , continue to cultivate for 36 hours.
[0056]...
Embodiment 3
[0064] Embodiment 3: Annexin V-FITC / PI double staining method detects formula 1a compound and formula 1b compound to MPP + Effects of induced apoptosis in human neuroblastoma SH-SY5Y cells.
[0065] 1. Cell culture steps are the same as in Example 1
[0066] 2. Cell grouping
[0067] The specific steps are the same as in Example 2, wherein the concentrations of the compound of formula 1a and the compound of formula 1b are both 12.5 μM.
[0068] 3. Annexin V-FITC / PI double staining
[0069] The apoptosis rate was detected by Annexin V-FITC and PI apoptosis detection kit. First stained with AnnexinV-FITC and then stained with PI for 15 minutes at room temperature. The apoptosis rate was quantified by flow cytometry (Becton Dickinson, Franklin Lakes, USA).
[0070] 4. Statistical processing is the same as in Example 2
[0071] 5. Experimental results
[0072] Experimental results such as Figure 14 As shown, compared with the control group, 1mM MPP alone + When treated, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com