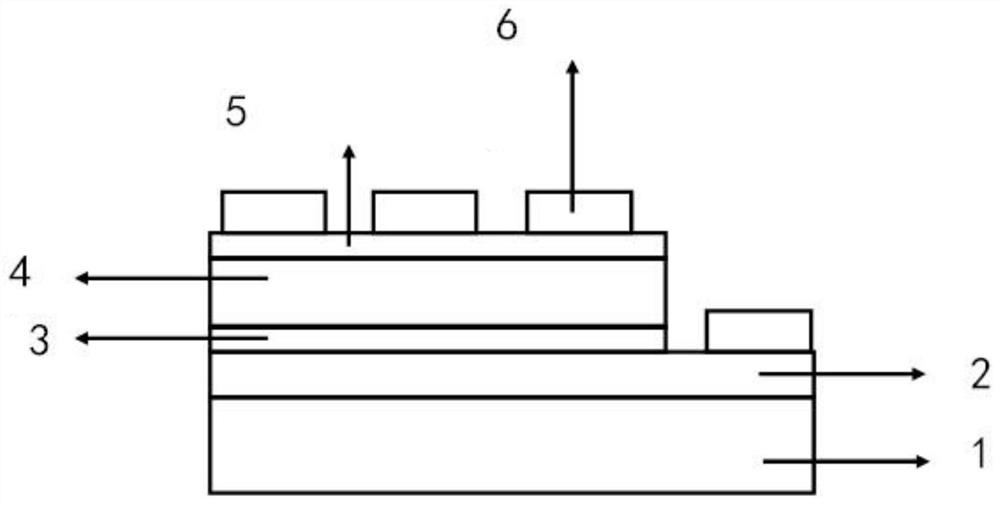
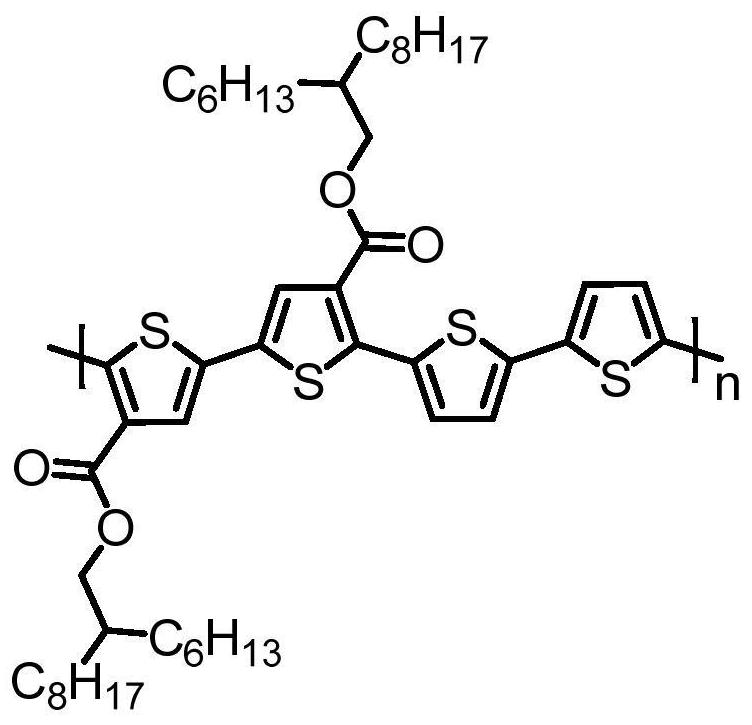
Chlorine-substituted polythiophene derivatives and solar cells
A technology of polythiophene derivatives and solar cells, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of low open circuit voltage, limited energy conversion efficiency, etc., and achieve the effect of high open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of raw materials M1 and M1',:
[0026]
[0027] (M1) and (M1'); wherein R is a branched alkyl chain in the formula (M1), and R is an alkyl chain in the formula (M1').
[0028] Preparation of raw materials N1(II-1)(II-4), N2(II-2)(II-5), N3(II-3)(II-6):
[0029]
[0030] Preparation of product:
[0031]
[0032]
[0033]
Embodiment 1
[0034] Embodiment 1: (2-butyloctyl)-2-bromothiophene-3-carboxylate
[0035]
[0036] 2-Bromo-3-carboxythiophene (1.50g, 7.28mmol), DCC (0.87g, 8.74mmol), DMAP (0.23g, 2.55mmol), 2-butyl-1-octanol (2.70g, 14.50mmol ) was dissolved in 50 mL of dichloromethane and stirred at room temperature for 24 hours. Then it was poured into water, filtered, and the filter cake was washed several times with dichloromethane. The filtrate was collected, washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the solvent was spin-dried, and the crude product was subjected to silica gel column chromatography (the eluent was petroleum ether (PE): CH 2 Cl 2 =6:1) Purified to obtain colorless liquid (2-butyloctyl)-2-bromothiophene-3-carboxylate. The structural characterization data are as follows: 1 H NMR (CDCl 3 ,400MHz): δ(ppm)=7.92(d,J=1.6Hz,1H),7.52(d,J=1.6Hz,1H),4.14(d,J=5.6Hz,2H),1.75-1.66(m ,1H),1.45-1.19(m,16H),0.85-0.90(m,6H).
Embodiment 2
[0037] Example 2: (2-hexyldecyl)-2-bromothiophene-3-carboxylate
[0038]
[0039] 2-Bromo-3-carboxythiophene (1.50g, 7.28mmol), DCC (0.87g, 8.74mmol), DMAP (0.23g, 2.55mmol), 2-hexyl-1-decanol (3.50g, 14.50mmol) Dissolve in 50 mL of dichloromethane and stir at room temperature for 24 hours. Then it was poured into water, filtered, and the filter cake was washed several times with dichloromethane. The filtrate was collected, washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the solvent was spin-dried, and the crude product was subjected to silica gel column chromatography (the eluent was petroleum ether (PE): CH 2 Cl 2 =6:1) Purified to obtain colorless liquid (2-hexyldecyl)-2-bromothiophene-3-carboxylate. The structural characterization data are as follows: 1 H NMR (CDCl 3 ,400MHz): δ(ppm)=7.94(d,J=1.6Hz,1H),7.54(d,J=1.6Hz,1H),4.17(d,J=5.6Hz,2H),1.78-1.68(m ,1H),1.46-1.19(m,24H),0.85-0.90(m,6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com