Therapeutically active complexes
A complex and active technology, applied in the direction of organic active ingredients, drug combinations, animal/human proteins, etc., can solve the problems of tissue toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0156] An α-helical, membrane-interacting peptide is tumor-killing when mixed with oleate
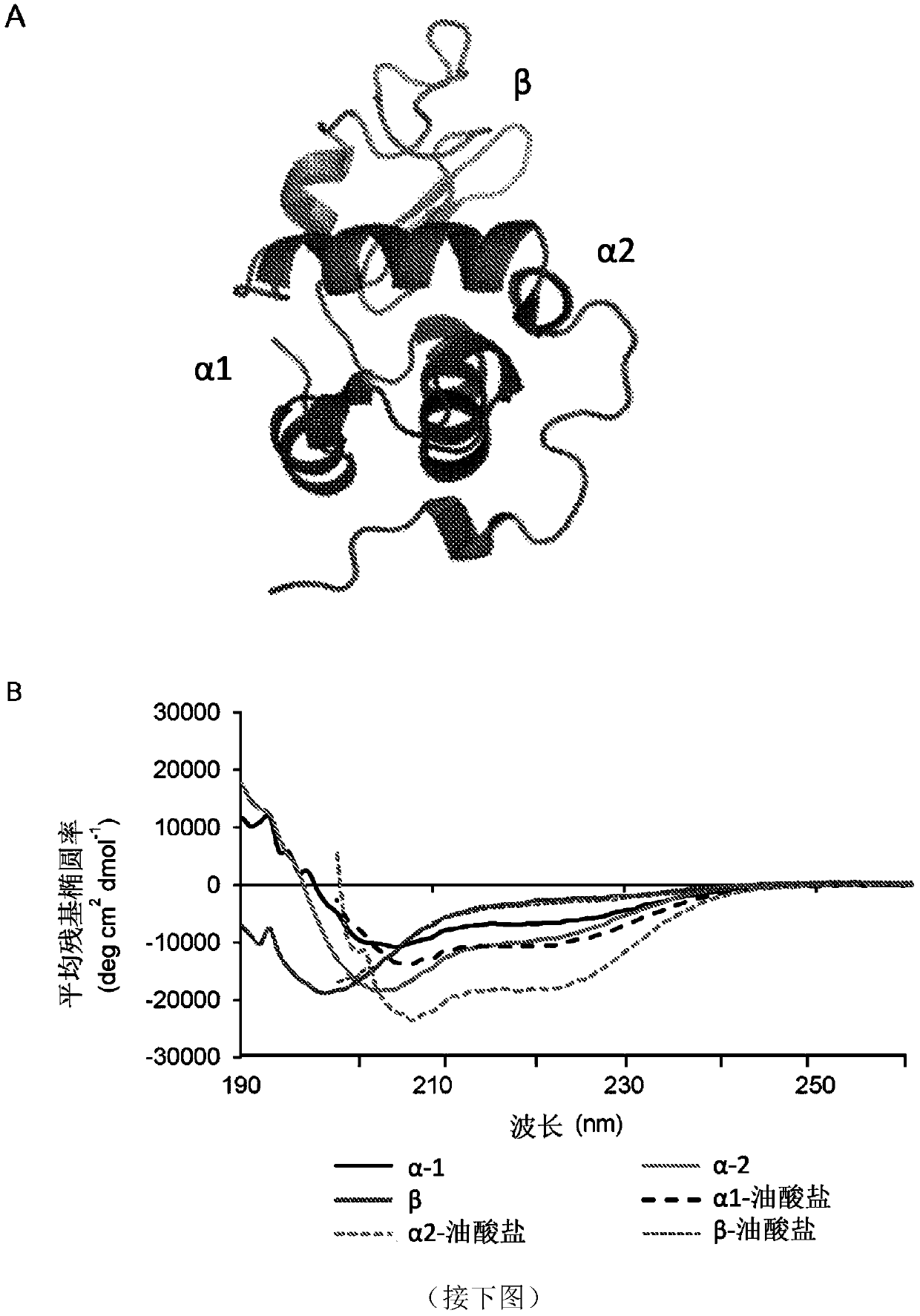
[0157] The above results suggest that α-lactalbumin acquires bactericidal activity by exposing membrane-interfering α-helical domains and binding oleate. To address that this 'gain of function' might be a general feature of membrane-interacting α-helical peptides, Sar1-based peptides—a member of the COPII complex—were also investigated. Like HAMLET, Sar1 alters membrane curvature and induces microtubulation through membrane insertion of an N-terminal amphipathic α-helix. The N-terminal α-helical peptide Sar1-α23 (SEQ ID NO 4) was therefore synthesized and compared with the Sar1-β46-78 peptide (SEQ ID NO 5) which forms a β-sheet in the native structure. Structural predictions and circular dichroism measurements indicate that Sar1-α is predominantly α-helical both with and without bound oleate. Sar1-β is partially helical and slightly more helical when bound to fatty acids (Fig. 6a-b an...
Embodiment 9
[0162] Therapeutic effect of Sar1α-peptide oleate complex in a mouse bladder cancer model.
[0163] In the above exemplary embodiment 6 and Figure 5The therapeutic activity of the Sar1α-oleate complex was demonstrated in the murine bladder cancer model described in A. This assessment has biological replicates and is investigator blinded. Tumor growth was attenuated in mice treated with sar1α-oleate, and bladder tissue was more intact than in sham-treated mice (P Figure 9 B-9F). Therapeutic efficacy is comparable to that of alpha 1-oleate (n.s., Figure 9 C-9D). Mice treated with sar1α-oleate or α1-oleate complex also showed significantly reduced expression of tumor proliferation markers cyclin D1, Ki-67 and VEGF as quantified by immunohistochemistry of frozen tissue sections ( Figure 9 E, 9F).
[0164] The results demonstrate that Sar1α-oleate is a second α-helical peptide-lipid complex with tumor specificity and therapeutic potential. The results suggest that certain...
Embodiment 10
[0166] Biomolecular NMR Analysis of Peptide-Oleate Complexes
[0167] To determine the structural prerequisites for this shared activity, the structural changes of the peptide upon conjugation to oleate were investigated by NMR spectroscopy. Native protein structure is generally considered a prerequisite for biological function. When proteins misfold, they lose biological activity and can form amorphous aggregates and amyloid fibrils that are toxic to host tissues. However, we have shown that in the presence of HAMLET there is tumor-killing activity when the protein is partially unfolded in a molten globular state. The molten globules retain secondary structural elements but lack tight packing inside, resulting in loss of overall tertiary structure. As observed by biomolecular NMR spectroscopy, the polypeptide backbone and side chains of these proteins are in conformational exchange, resulting in broad peaks and poor chemical shift dispersion ( Figure 10 ).
[0168] 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com