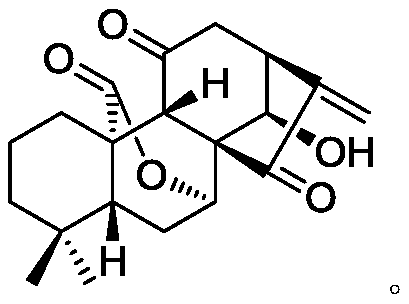
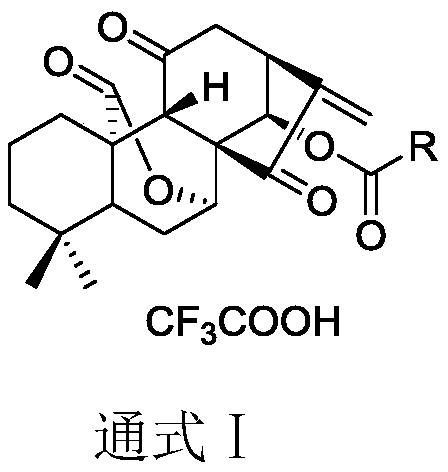
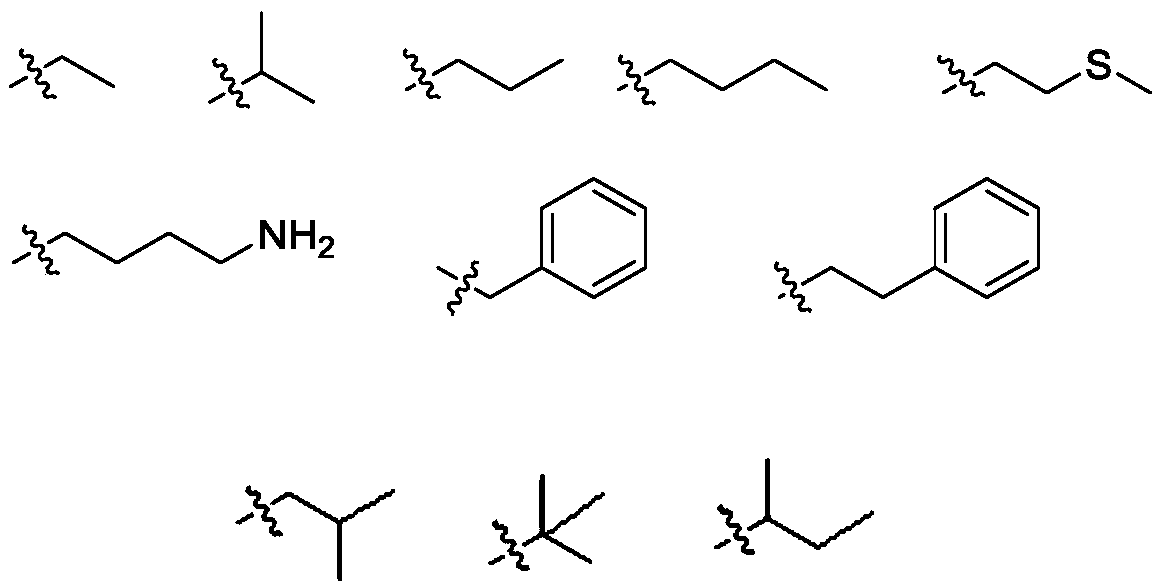
11, 20-dicarbonyl Jiyuan rubescensin a and L-amino acid-14-ester trifluoroacetate
A technology of ester trifluoroacetate and Rubescensin A, which is applied in the field of new-type Jiyuan Rubescensin A compounds, can solve problems such as instability, difficulty in intravenous administration, failure to reach blood drug concentration, etc., and achieve preparation The method is simple, the side effects of allergies are overcome, and the preparation method is easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of 11,20-Dicarbonyl Jiyuan Oridonin (Compound 2, JDAO)
[0035] Weigh 1 g of Jiyuan Rubescensine A (JDA), add 3.5 mL of acetone to dissolve it, and add an appropriate amount of Jones reagent dropwise to the system under stirring in an ice bath, and react for about 10 minutes. After the completion of the reaction was determined by thin-layer chromatography, the system was diluted with isopropanol, and the solvent was evaporated to dryness by rotary evaporation. After diluting with 50 mL of ethyl acetate, it was washed 5 times with water and stripped once. The ester layers were combined, dried with anhydrous sodium sulfate for 1 hour, and rotary evaporated to obtain 750 mg of JDAO (11,20-dicarbonyl oridonin, compound 2) as a white solid, with a yield of 95%.
Embodiment 2
[0037] Preparation of L-alanine-14-(11,20-dicarbonyl Jiyuan oridonin) ester trifluoroacetate (compound Ⅰ-1)
[0038] Weigh 150mg JDAO, dissolve it with 2mL dichloromethane, add Boc-L-alanine, EDCI (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride successively under stirring at room temperature ), DMAP (4-dimethylaminopyridine) and DIPEA (N,N-diisopropylethylamine). The reaction can be completed after 1-2 hours. After the completion of the reaction was determined by thin-layer chromatography, 30 mL of dichloromethane was added to dilute the reaction system, and the reaction system was washed three times with saturated ammonium chloride solution, and the combined water layers were back-extracted once with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate for 1 hour, and purified by column chromatography.
[0039] The resulting white solid was dissolved in dichloromethane and ice bathed. When the temperature of the system dropped...
Embodiment 3
[0041] Preparation of L-valine-14-(11,20-dicarbonyl Jiyuan oridonin A) ester trifluoroacetate (compound I-2)
[0042] Weigh 150mg of JDAO, dissolve it in 2mL of dichloromethane, add Boc-L-valine, EDCI, DMAP and DIPEA successively under stirring at room temperature. The reaction can be completed after 1-2 hours. After the completion of the reaction was determined by thin-layer chromatography, 30 mL of dichloromethane was added to dilute the reaction system, and the reaction system was washed three times with saturated ammonium chloride solution, and the combined water layers were back-extracted once with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate for 1 hour, and purified by column chromatography.
[0043] The resulting white solid was dissolved in dichloromethane and ice bathed. When the temperature of the system dropped to 5°C, a mixed solution of dichloromethane:trifluoroacetic acid 1:1 was added to the system, and stirred in an i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com