Nanoparticle formulations
A technology of nanoparticles and formulations, applied in the fields of nanomedicine, nanotechnology, nanotechnology, etc., can solve problems such as adverse effects of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0170] Example 1: Construction of the TAT-MYC fusion peptide of the present technology
[0171] Plasmid pTAT-MYC-V5-6xHis was made by PCR amplification of the coding region of human MYC using a forward primer containing in frame the TAT protein transduction domain of HIV-1 and a reverse primer The inner N-terminal 10-amino acid sequence (MRKKRRQRRR (SEQ ID NO: 7), and the reverse primer removes the stop codon. The PCR product is cloned into the pET101 / D-Topo (Invitrogen) vector, which contains the C-terminal V5 Epitope tags and 6-histidine protein tags.
[0172] A. Bacterial strains for protein expression
[0173] By transforming BL-21Star with pRARE(CamR) isolated from BL21 Rosetta cells (Novagen) TM Escherichia coli strain (Invitrogen) was used to establish BL-21RARE cells expressing tRNAs of AGG, AGA, AUA, CUA, CCC, GGA codons.
[0174] B. Protein Induction and Purification
[0175] To prepare fermenter inoculum, vials of TAT-MYC master cell bank (MCB) were thawed and...
example 2
[0179] Example 2. Preparation of Nanoparticle TAT-MYC Compositions
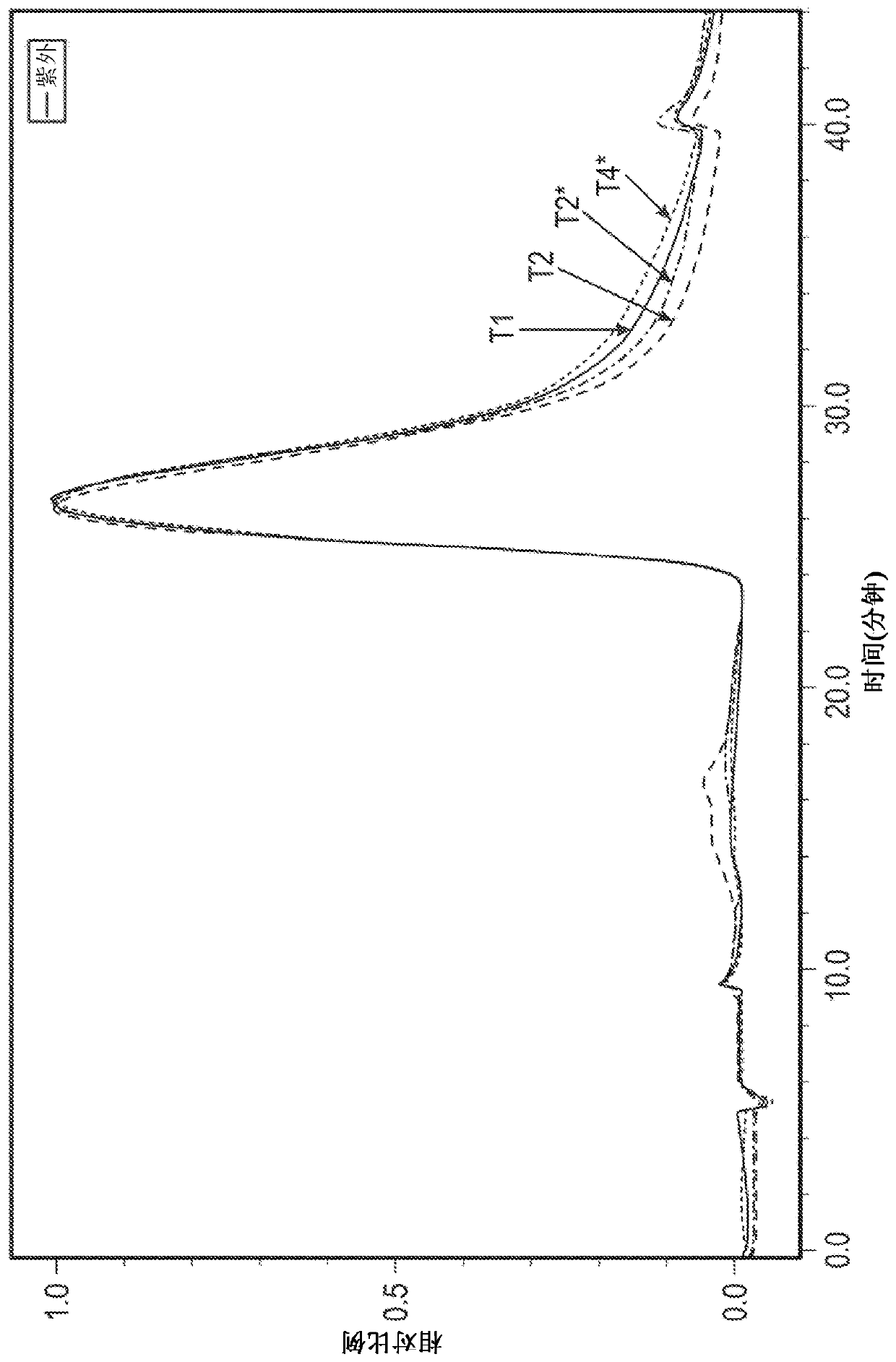
[0180] Refolding of the TAT-MYC protein in the Q-Sepharose flow-through pool from Example 1 was accomplished using a tangential flow filtration-based refolding method using a UFDF (ultrafiltration / diafiltration) membrane. The refolding process consists of a series of three refolding steps. The first refolding step involves changing refolding buffer 1 (3M urea, 50 mM phosphate, 500 mM NaCl, 10% glycerol, 5 mM GSH (reduced glutathione), 1 mM GSSG (oxidized glutathione)). The second refolding step involves changing refolding buffer 2 (1.5M urea, 50mM phosphate, 500mM NaCl, 10% glycerol, 5mM GSH (reduced glutathione), 1mM GSSG (oxidized form) over the course of approximately 120 minutes glutathione)), followed by about 120 minutes of recirculation. The third refolding step consists of changing refolding buffer 3 (50 mM phosphate, 500 mM NaCl, 10% glycerol, 5 mM GSH (reduced glutathione), 1 mM GSSG (oxidized ...
example 3
[0184] Example 3. Functional Analysis of Nanoparticle TAT-MYC Compositions
[0185] The activity of TAT-MYC compositions was tested as follows.
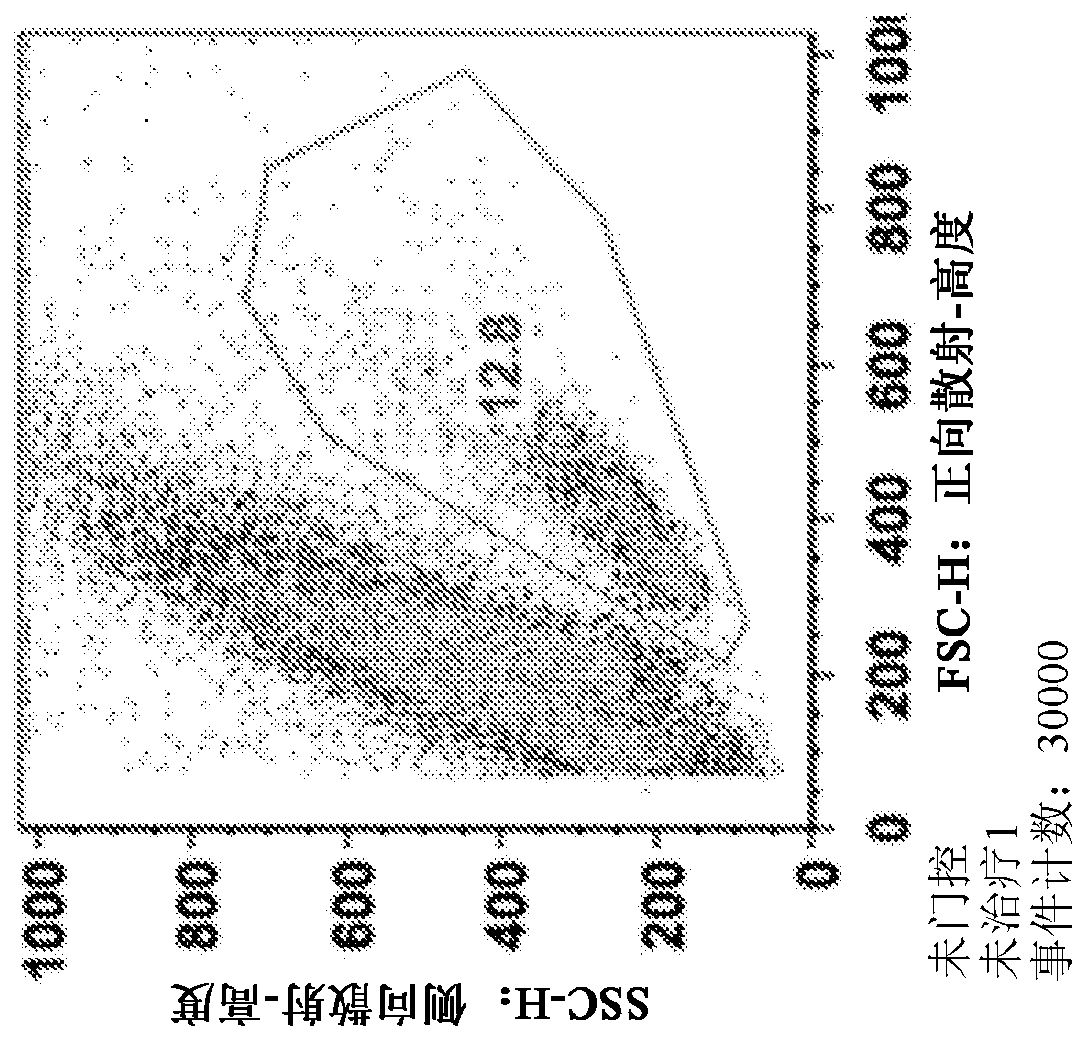
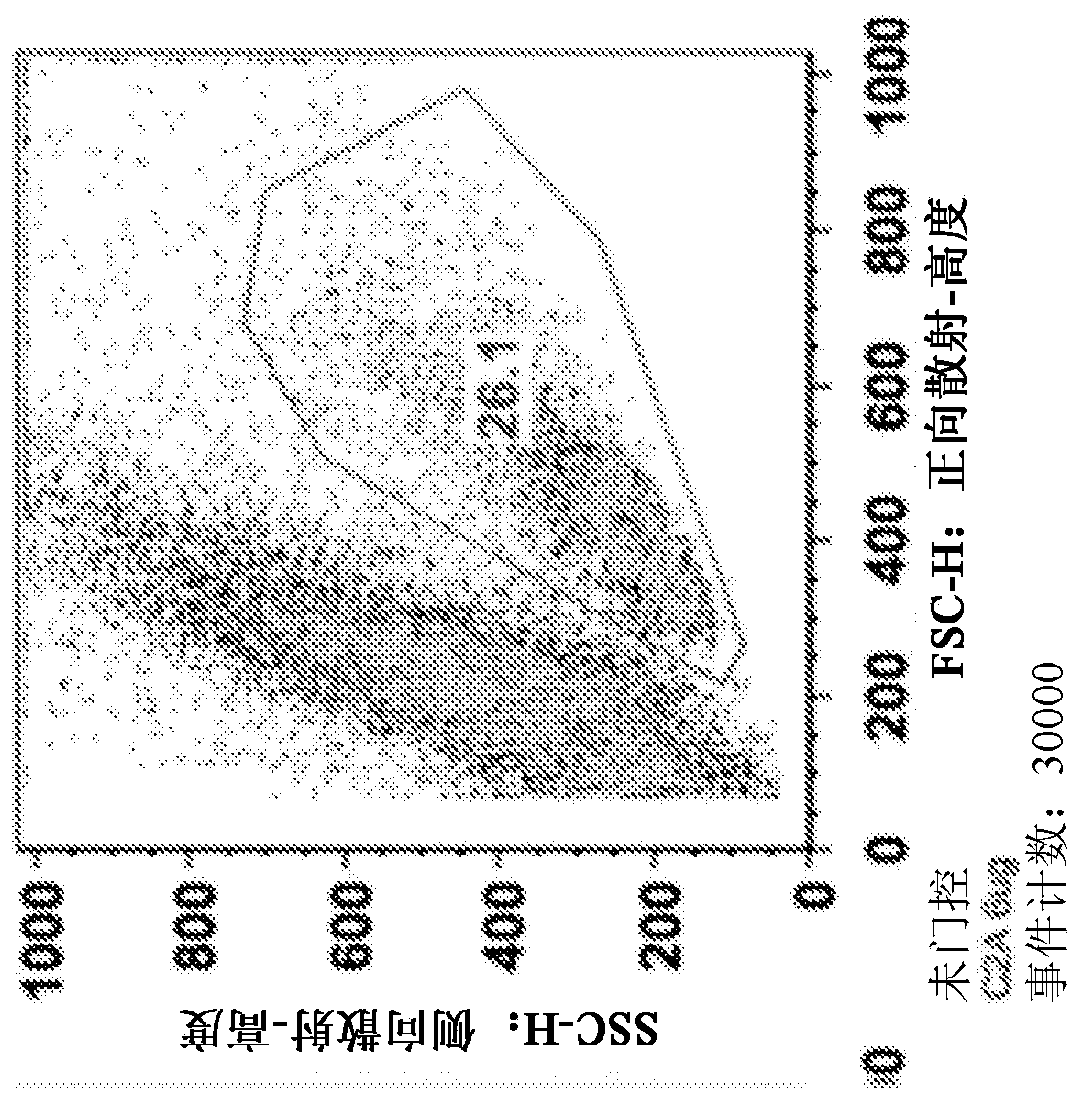
[0186] Spleens were harvested from C57BL / 6j (Jackson) mice and mechanically dissociated through wire mesh. Red blood cells were removed, CD4 positive T cells were isolated using a commercially available isolation process (Dynabead), and T cells were activated with 1 μg / ml anti-CD3 and anti-CD28 antibodies. Cells were plated at 1.5x10 per well in 1ml medium 6 cells were seeded in 48-well cluster culture dishes. After 24 hours, the TAT-MYC formulation was added to the cells (total 12 μg per well) and incubated for 24 hours. Twenty-four hours after protein addition, the medium was changed and cells were incubated for 48 hours. Cell viability was assessed 96 hours after initial activation via flow cytometry (forward x side scatter). The results for C2A (prepared according to Example 2) are shown in Figure 1 .
[0187] Figure 1A ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com