Optimized synthetic consensus inmunogenic compositions targeting fibroblast activation protein
A technology of immunogenicity and immunogenic fragments, applied in the field of immunogenic compositions, can solve problems such as lethal toxicity and slowing down of tumor progression by T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1: Synthetic Consensus FAP Immunogenic Compositions
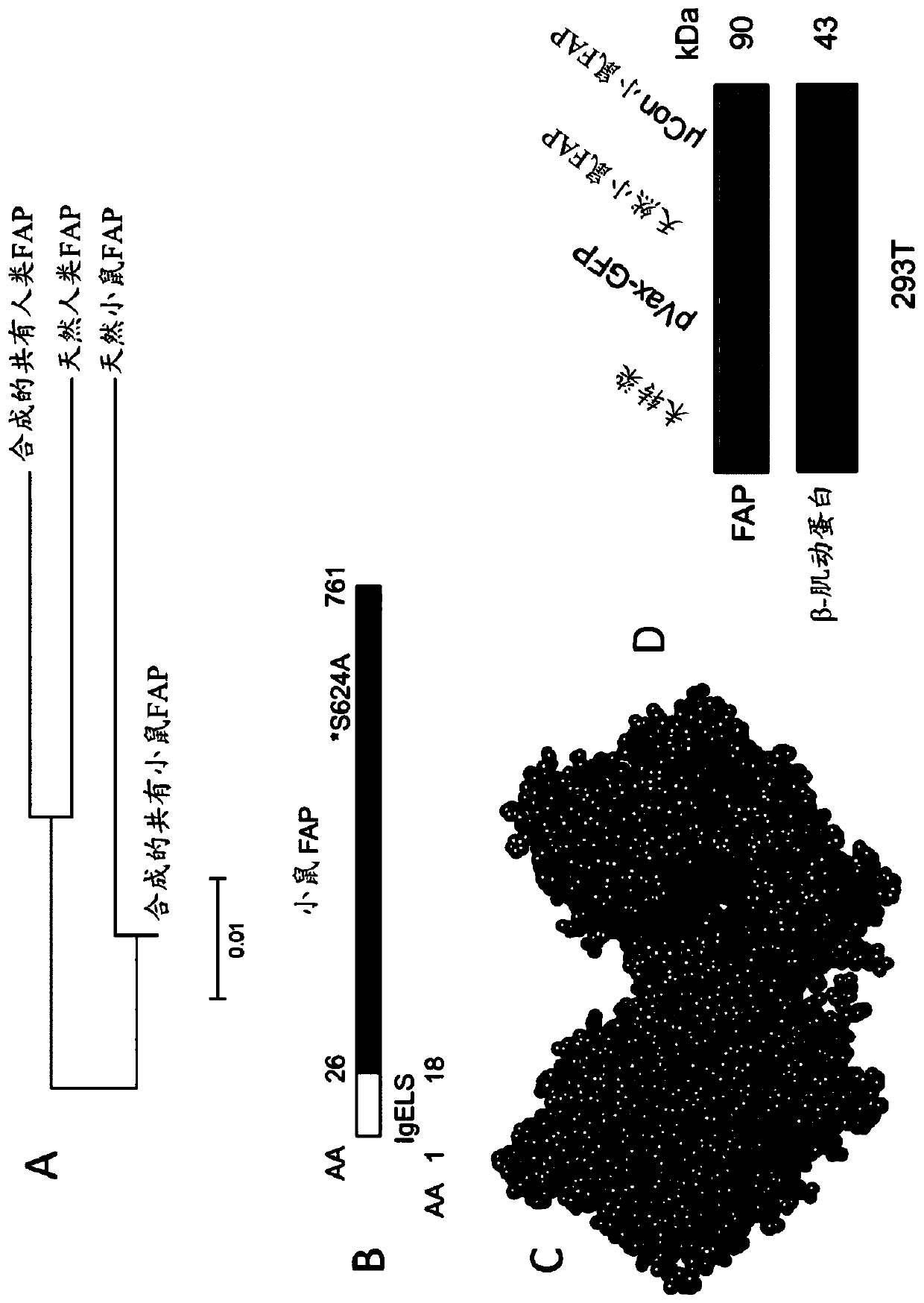
[0245] The FAP protein is a protease and gelatinase expressed on activated fibroblasts. FAP is expressed in >90% of cancer-associated fibroblasts in human cancers, including in prostate and pancreatic cancers. FAP is also expressed in fibroblasts involved in wound healing and in malignant cells of bone and soft tissue sarcomas. Antibodies against FAP (eg, sibulizumab) and small molecule inhibitors of FAP (eg, talabostat) are safe but have shown minimal efficacy in clinical trials.
[0246] Over the past decade, there has been a surge of interest in developing immunotherapies targeting FAP-expressing cells (Fang et al., 2016, Mol Ther Oncolytics, 3:16007; Gottschalk et al., 2013, PLoS One, 8:e82658; Zhang and Ertl, 2016, Oncotarget, 7:23282–99; Xia et al., 2016, Cancer Immunol Immunother, 65:613–624; Wen et al., 2010, Cancer Sci, 101:2325–2332; Xia et al., 2016, 34 :4526-4535; Chen et al., 2015, Sci Rep, ...
Embodiment 2
[0297] Example 2: Immunogenic Fragments of FAP
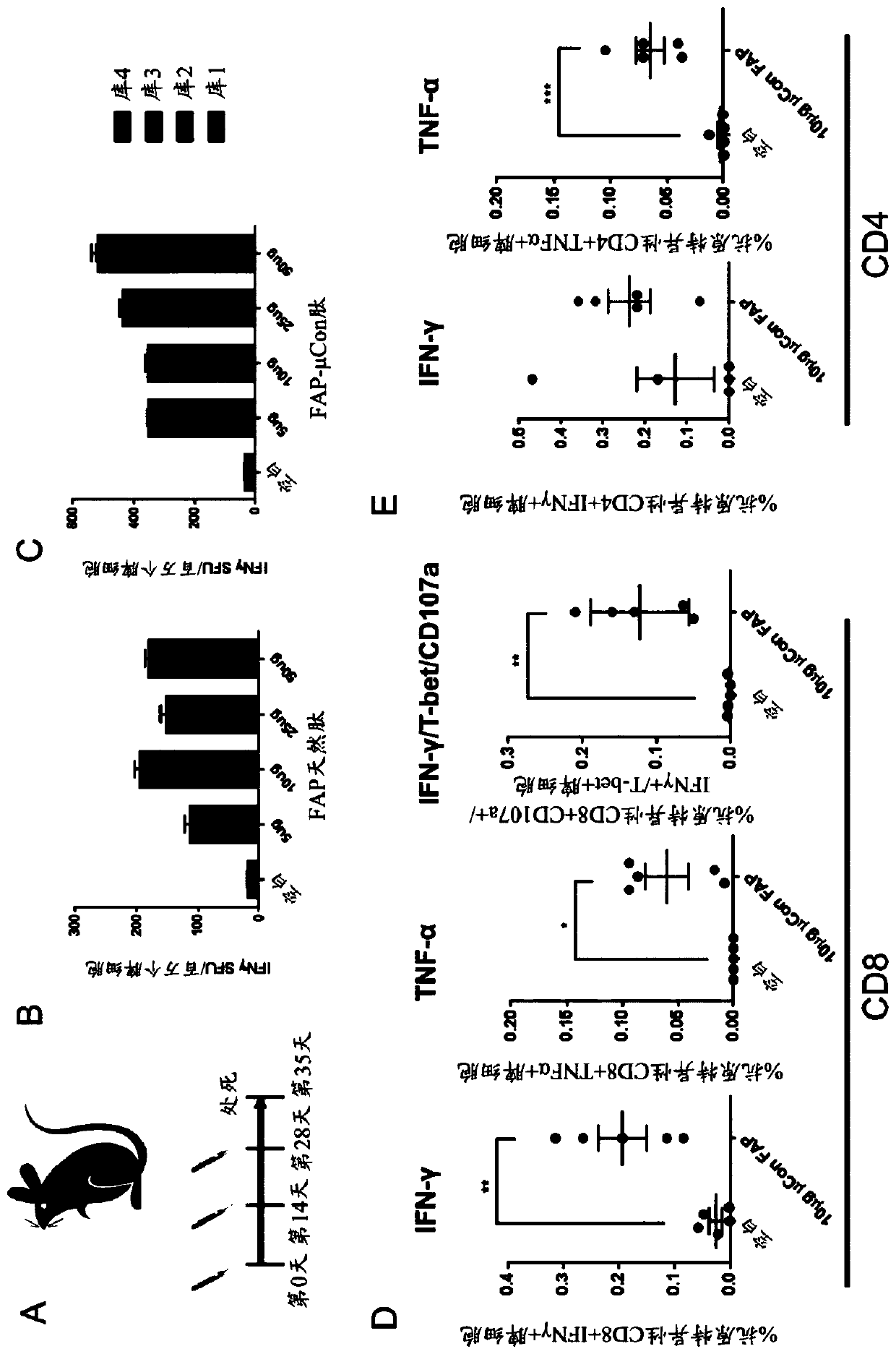
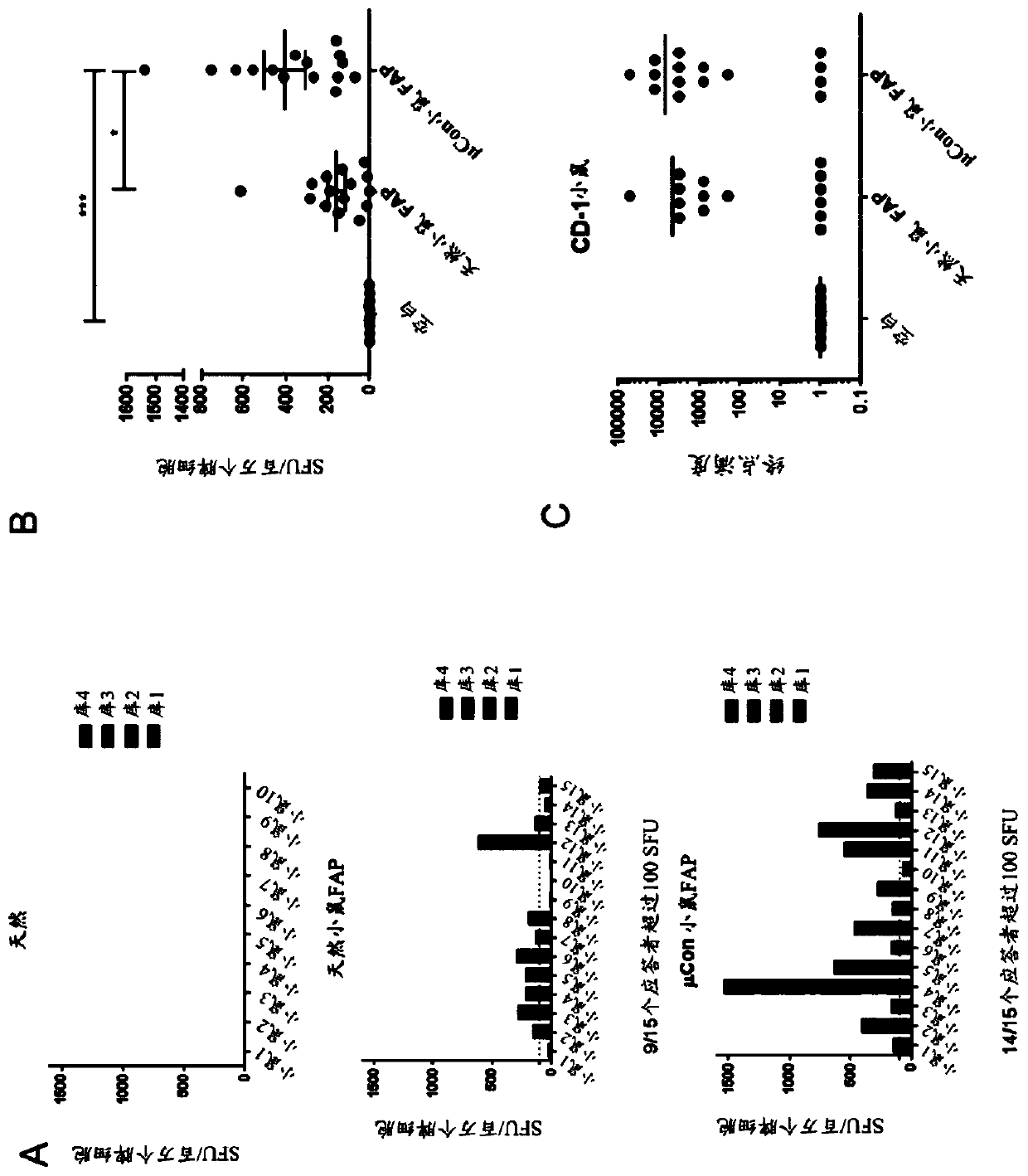
[0298] To characterize the response of mouse strains to native FAP peptides and to determine dominant epitopes, 122 peptides representing different epitopes of FAP were generated for the optimized consensus FAP (Figure 14A). When cells were stimulated with each pool, there were multiple responses (Figure 14B and Figure 14C), leading to the conclusion that there is not one dominant epitope but multiple subdominant epitopes ( Figure 15 ). Several subdominant epitopes (e.g., SEQ ID NO:22, SEQ ID NO:23, and SEQ ID NO:24) contained mutations in the optimized consensus FAP relative to native FAP ( Figure 15 ).
Embodiment 3
[0299] Example 3: Sequence Information
[0300]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com