Ergosterol compounds with anti-inflammatory activity, and preparation method and application thereof
An ergosterol and compound technology, applied in the preparation of anti-inflammatory drugs, the ergosterol compound and the preparation field thereof, can solve the problems such as no ergosterol compound NO inhibitor, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Separation and purification of compounds:
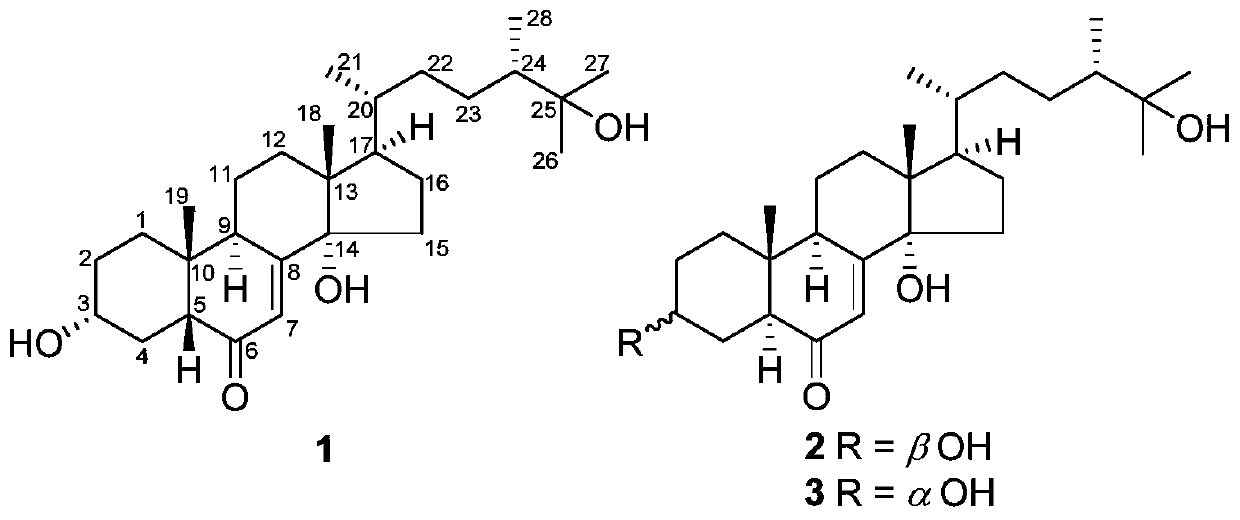
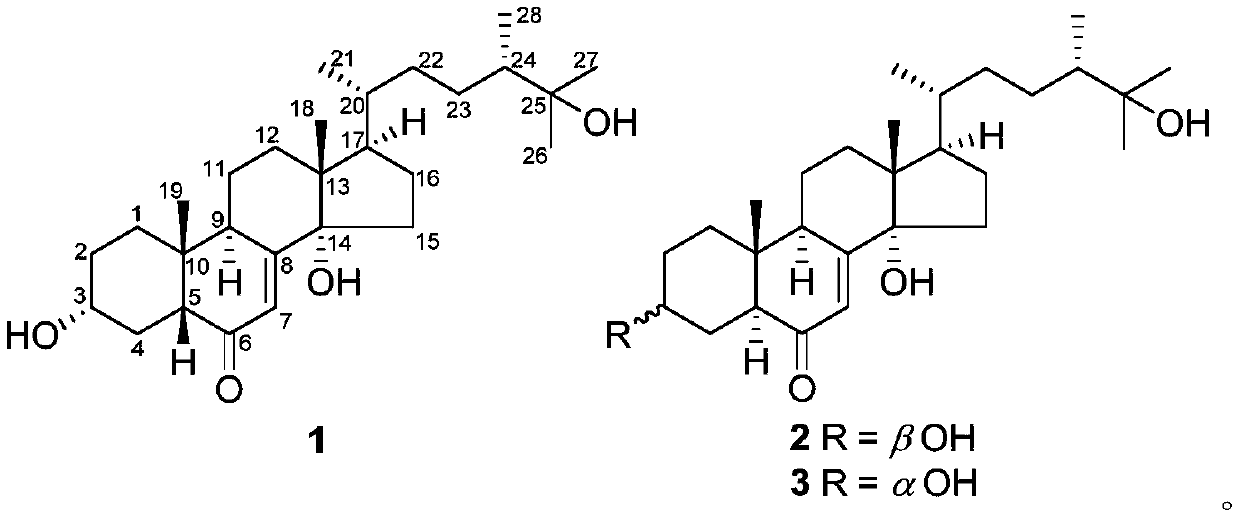
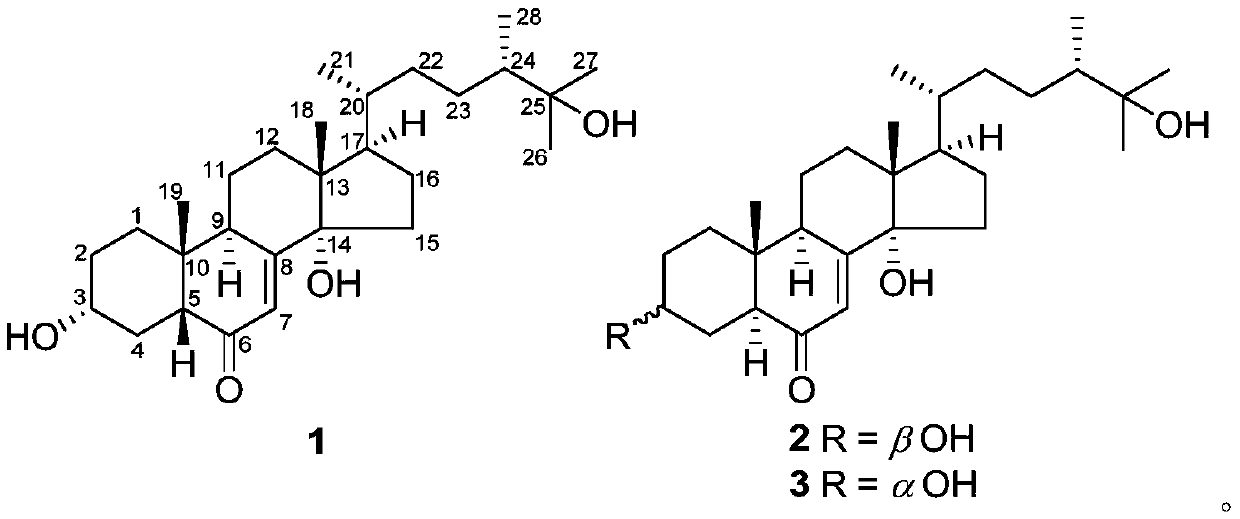
[0028] The method for preparing the compound, crushing the fruit body of Zizhi lucidum, extracting with 95% ethanol under reflux for 2 hours, combining the extracts and recovering the crude extract of the solvent under reduced pressure, suspending the crude extract in water, extracting three times with an equal volume of ethyl acetate, combining the extraction The ethyl acetate extract concentrated under reduced pressure, the extract was subjected to silica gel 200-300 mesh column chromatography, chloroform / methanol system (100:1,50:1,20:1,10:1,5:1, 2:1, 1:1 and 0:1 v / v) gradient elution, and 7 fractions were collected. Fraction C6 was subjected to reverse phase RP-18 column chromatography, eluting with 10%-100% methanol / water to obtain 4 sub-fractions. The fraction of C6-3 was analyzed by HPLC (MeCN / H 2 0,20%-60%, 8mL / min) to obtain compound 1 (6mg, t R =12.4min), 2(4mg,t R =18.3min), and 3(2mg,t R = 20.1 min).
[0029]...
Embodiment 2
[0042] Anti-inflammatory activity test
[0043] The monomeric compounds 1-3 acted on lipopolysaccharide (LPS)-induced RAW264.7 mouse macrophages respectively, and the NO level in the culture supernatant was detected by the Griess reagent chromogenic method to determine the ability of the test drugs to inhibit the release of NO As a screening index, the anti-inflammatory activity of the compound was evaluated.
[0044] 1. Preparation of drug solution:
[0045] (1) The monomer compounds 1-3 were respectively prepared into 100 μg / ml solutions with DMSO, and stored at -4°C;
[0046] (2) LPS: E.Coli O111:B4 (Merck Millipore, USA, product number: LPS25), made into a 100 μg / mL solution with redistilled water, and stored at -20°C.
[0047] 2. Cell line: RAW264.7 mouse macrophages, provided by the Shanghai Branch of the Chinese Academy of Sciences.
[0048] 3. Test method:
[0049] (1) A single mouse macrophage suspension was prepared with 10% fetal calf serum culture medium, and t...
Embodiment 3
[0058] Preparation of injection preparations:
[0059] First obtain compounds 1, 2, and 3 of the present invention according to the method of Example 1, take 50 mg of one or several compounds, dissolve it in 2 ml of propylene glycol, filter the resulting solution and pack it into an ampoule under aseptic conditions .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com