Quantitative measurement method for basic lanthanum carbonate impurities in lanthanum carbonate chewable tablets
A technology of lanthanum carbonate and chewable tablets, which is applied in measuring devices, material analysis using radiation diffraction, instruments, etc., can solve the problems of excipient interference and cumbersome result processing, and achieve good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] 1) Preparation of the test product: Take 10 lanthanum carbonate chewable tablets, grind them, mix them evenly, take the powder through a 100-mesh sieve, accurately weigh 1261 mg of the sieved fine powder, containing 577.2 mg of lanthanum carbonate, and weigh two in parallel ; Take a fine powder as the test product;
[0033] 2) Preparation of reference substance: Take another portion of the fine powder and add 24.95 mg of lanthanum basic carbonate crystal form I passing through a 100 mesh sieve, and 27.72 mg of lanthanum basic carbonate crystal form II passing through a 100 mesh sieve, and mix well. Reference substance
[0034] 3) Measurement and calculation: Measured by X-ray diffraction method, using Cu target as light source, θ~2θ linkage continuous scanning, scanning interval 0.1, scanning rate 0.4s / step, and the diffraction angle 2θ is 15°~50° In a single scan, the test article and the reference substance were measured, and each sample was measured 3 times in parallel; ...
Embodiment 1
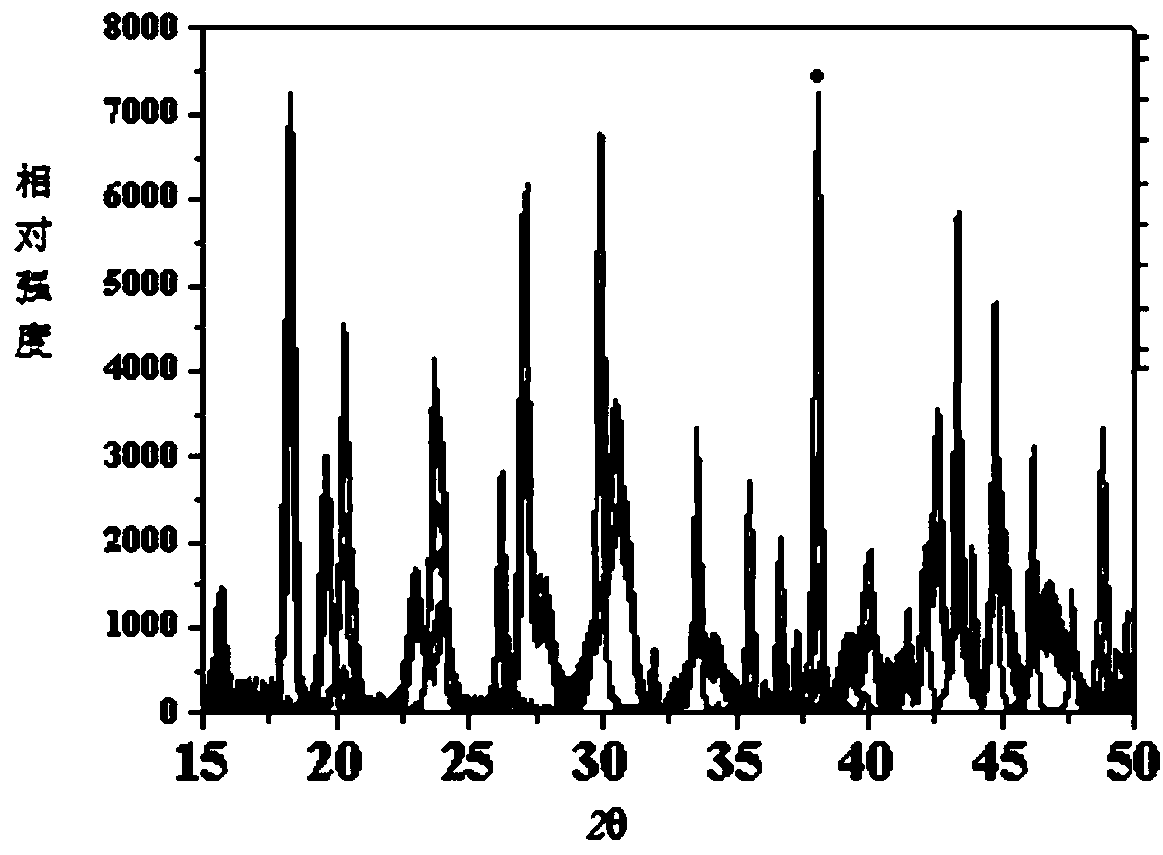
[0035] Example 1 Specificity study
[0036] Blank excipients: directly take samples of blank excipients (glucose, silicon dioxide, and magnesium stearate prepared according to the mass ratio of 1:1:1), pass a 100-mesh sieve, and take the sieved sample.
[0037] Raw material medicine: Take a sample of the raw material medicine (lanthanum carbonate) directly, pass a 100-mesh sieve, and take the sieved sample.
[0038] Preparation: Take this product (lanthanum carbonate chewable tablet, 500mg specification), grind it through a 100 mesh sieve, and take the sieved sample.
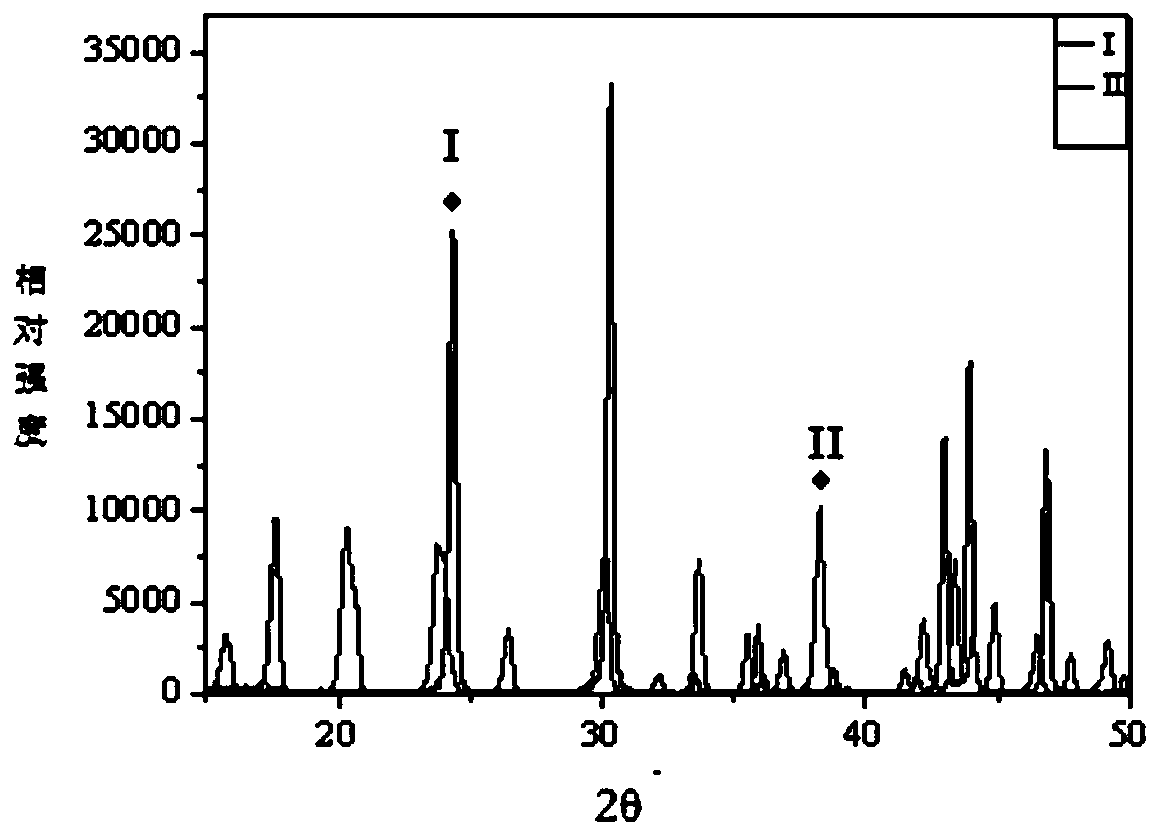
[0039] Basic lanthanum carbonate crystal form I: Take the basic lanthanum carbonate crystal form I reference substance (reference substance level, this sample is used in the experimental scheme of the present invention) after a 100-mesh sieve.
[0040] Basic lanthanum carbonate crystal form II: Take the basic lanthanum carbonate crystal form II reference substance (reference level, this sample is used in the experimental ...
Embodiment 2
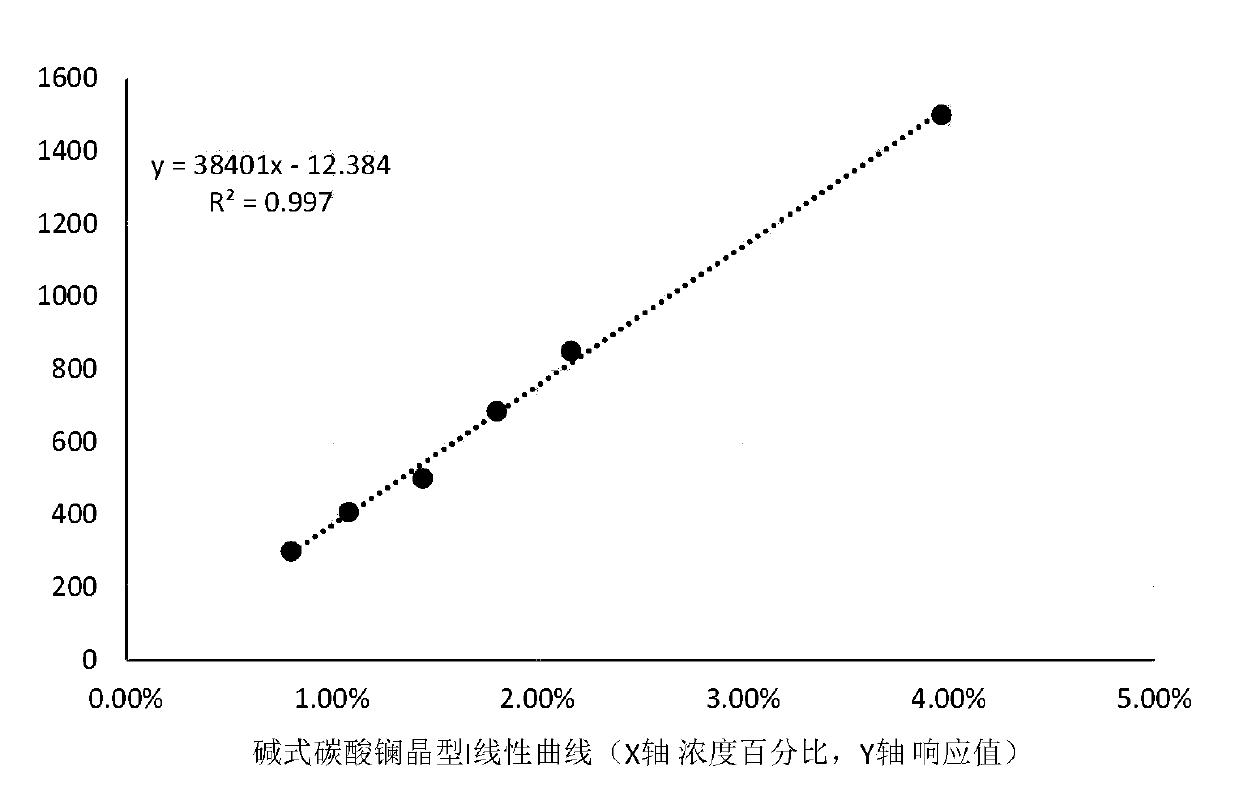
[0046] Example 2 Linear study
[0047] Take 15 pieces of this product (lanthanum carbonate chewable tablets, 500mg specifications) and grind them through a 100-mesh sieve. Take the fine powder of this product after sieving and proceed as follows.
[0048] Test product: Take 1261 mg of fine powder of this product (equivalent to 577.2 mg of lanthanum carbonate).
[0049] Quantitative limit concentration + preparation: take this product fine powder 1261mg (equivalent to 577.2mg of lanthanum carbonate), add basic lanthanum carbonate I4.70mg, basic lanthanum carbonate II 5.29mg, mix the above powders uniformly, determine (crystal form I 0.8 %, Form II 0.9%)]
[0050] Limit concentration 60% + preparation: take this product fine powder 1261mg (equivalent to lanthanum carbonate 577.2mg), add basic lanthanum carbonate I6.38mg, basic lanthanum carbonate II 7.09mg, mix the above powders uniformly, determine (crystal form I 1.08%, crystal form II 1.2%).
[0051] Limit concentration 80% + prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com