A class of quinoline fluorescent compounds, preparation method and application thereof
A technology of fluorescent compounds and quinolines, which is applied in the field of fluorescent dyes, can solve problems such as low excitation wavelength, short excitation and emission wavelength, and limit the application of quinoline, and achieve large displacement values, high quantum yields, and excellent fluorescence properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of 2--Chloro-7-diethylaminoquinoline-3-carbaldehyde (1)
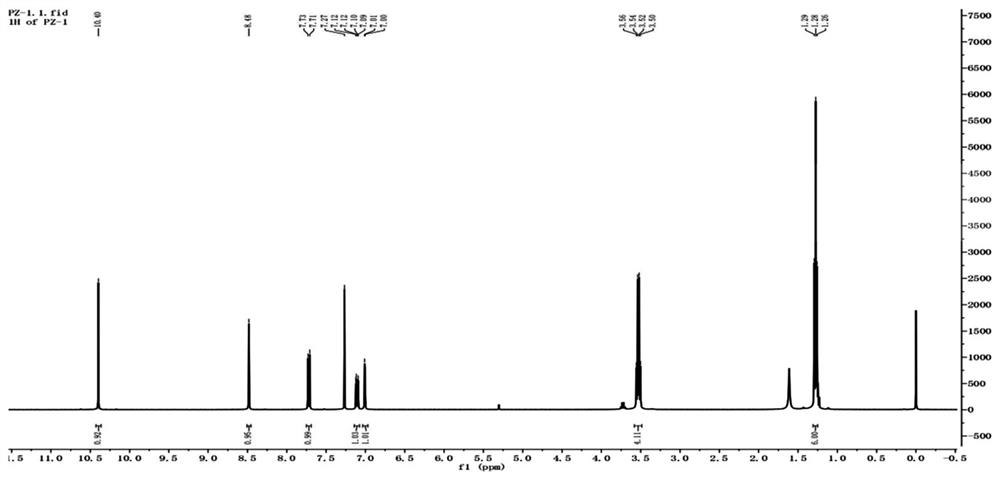
[0050] In the double-necked flask of 50ml, add 1.00g (4.8mmol) 2-diethylaminoacetanilide, 0.9g (4.7mmol) DMF, add 2.5g (8.4mmol) solid phosgene in the single-necked flask of 50mL, add 5mL1, 2-dichloroethane, so that the solid phosgene is completely dissolved, and the solid phosgene is added dropwise under an ice-salt bath. Half an hour was added dropwise. 60°C reaction. Followed by TLC, the reaction was complete. The reaction solution was poured into 50 mL of water, hydrolyzed for 5 min, spin-dried the organic solvent, suction filtered, dried, recrystallized from toluene, suction filtered and dried to obtain 0.82 g of a yellow-brown solid. Yield 66%. 1 H NMR (400MHz, Chloroform-d) δ 10.40 (s, 1H), 8.48 (s, 1H), 7.72 (d, J=9.2Hz, 1H), 7.11 (dd, J=9.2, 2.6Hz, 1H) ,7.01(d,J=2.5Hz,1H),3.53(q,J=7.1Hz,4H),1.28(t,J=7.1Hz,6H).
Embodiment 2
[0052] Preparation of 2-chloro-7-diethylaminoquinoline-3-carbonitrile (2)
[0053] Add 0.30 g (1.1 mmol) of 3-chloro-7-diethylaminoquinoline-3-carbaldehyde to the single-necked flask, add 3 mL of tetrahydrofuran to dissolve, add 0.5 g of iodine (1.9 mmol), 7 mL of ammonia water, stir at room temperature for 3-5 h, TLC tracking, when the reaction was completed, the reaction solution was poured into water, suction filtered, and dried. Silica gel column chromatography, eluent: petroleum ether: ethyl acetate (100:0-10, v / v), to obtain 0.24 g of a yellow solid. Yield: 80%. IR(KBr)cm -1 : 2215(C≡N), 1620(C=N), 688(C-Cl).
Embodiment 3
[0055] Preparation of 2-chloro-7-diethylaminoquinoline-3-carboxylic acid (3)
[0056] Add 0.20 g (0.8 mmol) of 3-chloro-7-ethylenediaminoquinoline-3-carbonitrile to a single-necked flask, add 2 mL of acetic acid, 5 mL of 70% sulfuric acid, heat under reflux for 2-3 h, TLC traces to after the reaction is complete, The reaction solution was poured into water, filtered with suction, the obtained solid was dissolved in 1,2-dichloroethane, 0.25 g (0.84 mmol) of solid phosgene was added, and a drop of DMF was refluxed for 3 to 5 h, followed by TLC until the reaction was completed, and a silica gel column Chromatography, eluent: petroleum ether: ethyl acetate, 100:0-50, v / v), eluted to obtain 0.18g of yellow-green solid, which is the product 2-chloro-7-diethylaminoquinoline-3- Formic acid (3). Yield: 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com