Method for carrying out quality control operation on atrial fibrillation single disease follow-up visit data
A technology for controlling operation and single disease, which is applied in the field of quality control operation of follow-up data of atrial fibrillation single disease, which can solve the difficulty of data quality control, the degree of redundancy of data integrity and the lack of solution to data quality control. and other problems, to achieve the effect of improving management efficiency, facilitating clinical management work, efficient collection and management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] In order to make the purpose, technical solutions and advantages of the embodiments of the present invention clearer, the technical solutions in the embodiments of the present invention will be clearly and completely described below in conjunction with the drawings in the embodiments of the present invention. Obviously, the described embodiments It is a part of embodiments of the present invention, but not all embodiments. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
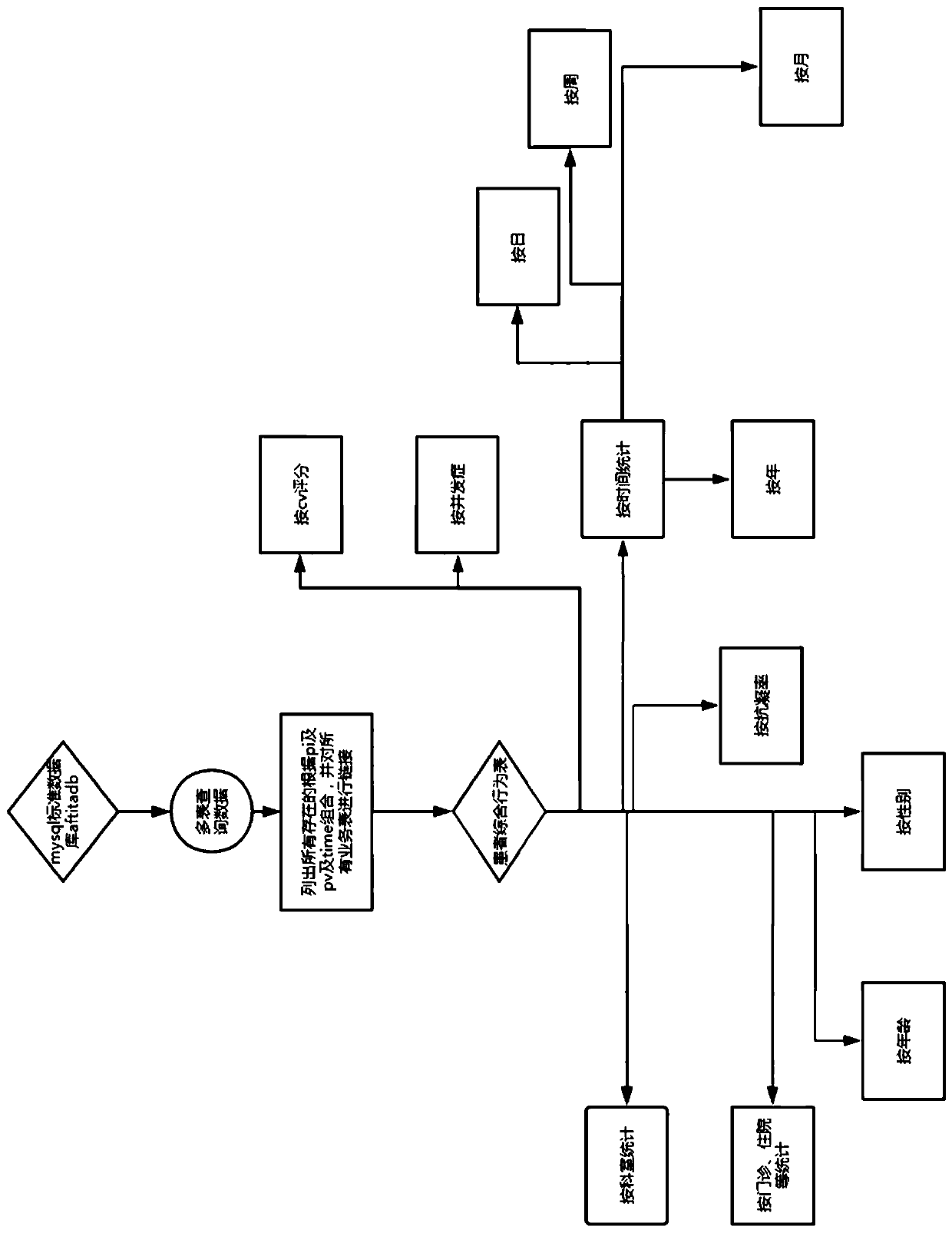
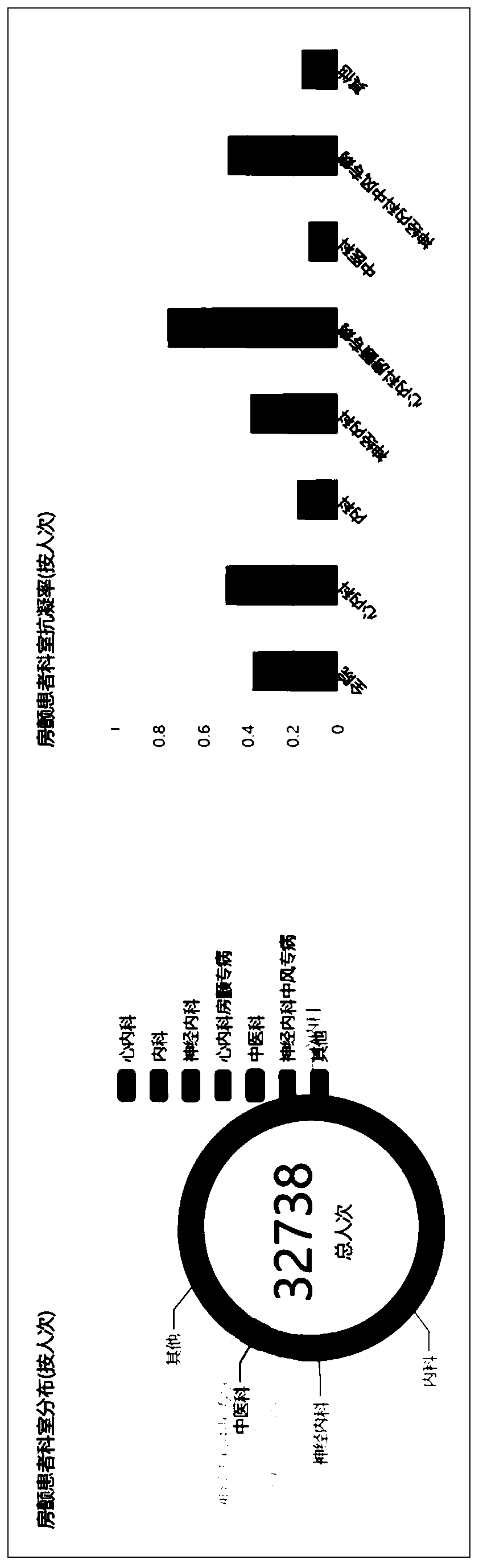
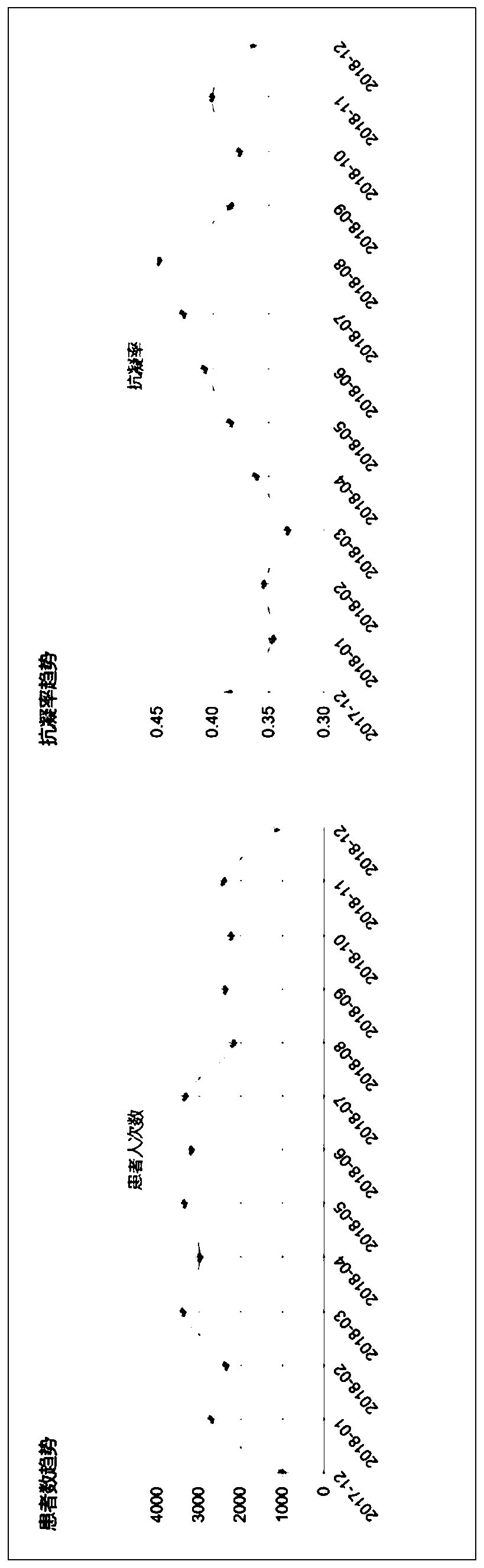
[0019] see Figure 1 to Figure 5 , in view of the difficulty in quality control existing in the processing of data in the prior art, after collecting and cleaning the data, this embodiment further conducts a series of follow-up data on the obtained high-quality atrial fibrillation single disease Quality control operations, including anticoag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com