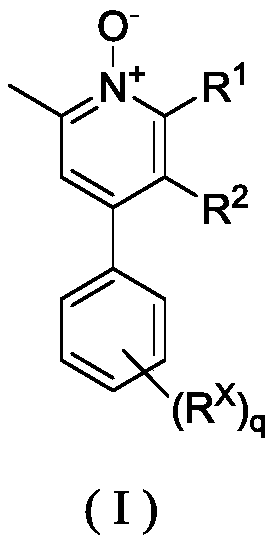
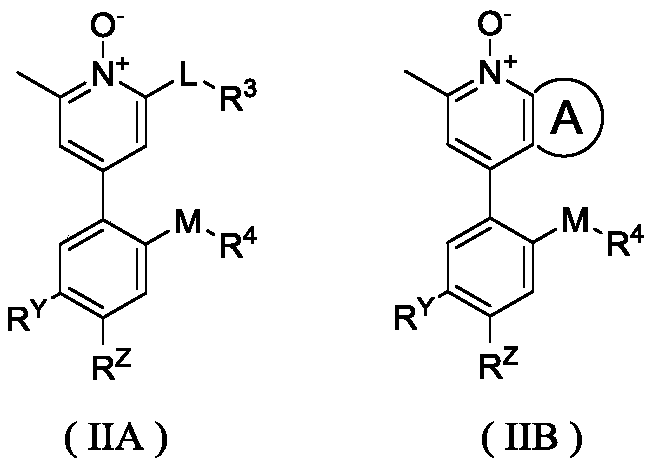
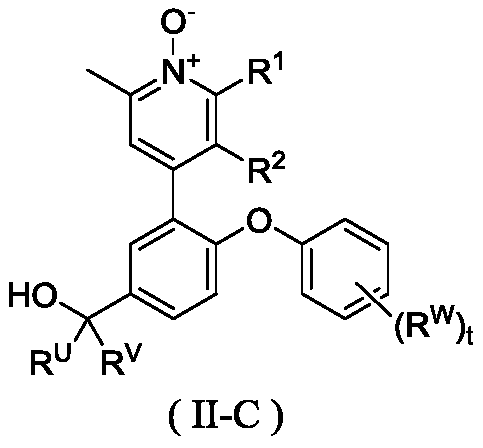
Pyridine N-oxidation derivative as well as preparation method and application thereof
A technology of derivatives and compounds, used in drug combination, organic chemistry, digestive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0216] Compound structures were determined by nuclear magnetic resonance (NMR) or mass spectroscopy (MS). The mensuration of NMR is to use BrukerAVANCE-400 nuclear magnetic instrument, and measuring solvent is deuterated dimethyl sulfoxide (DMSO-d 6 ), deuterated chloroform (CDCl 3 ), deuterated methanol (CD 3 OD), the internal standard is tetramethylsilane (TMS), and the chemical shift is 10 -6 (ppm) is given as a unit.
[0217] MS was determined with a FINNIGAN LCQAd (ESI) mass spectrometer (manufacturer: Thermo, model: Finnigan LCQadvantage MAX).
[0218] The determination of HPLC uses Agilent 1200DAD high pressure liquid chromatography (Sunfire C18 150×4.6mm chromatographic column) and Waters 2695-2996 high pressure liquid chromatograph (Gimini C18 150×4.6mm chromatographic column).
[0219] Kinase average inhibition rate and IC 50 Values were measured with a NovoStar microplate reader (BMG, Germany).
[0220] Use Yantai Huanghai HSGF254 or Qingdao GF254 silica gel...
Embodiment 1
[0302] 4-(2-(2,4-difluorophenoxy)-5-(ethylsulfonylamino)phenyl)-2,6-lutidine 1-oxidation
[0303]
[0304] The first step: the preparation of 2,6-dimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
[0305]
[0306] 4-bromo-2,6-lutidine (1.0g, 5.38mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'- Bi(1,3,2-dioxaborolane) (1.6g, 6.45mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (393mg, 0.54mmol) , Potassium acetate (1.6g, 16.12mmol) was dissolved in 1,4-dioxane (15mL), replaced with nitrogen, heated to 85°C and stirred overnight. LC / MS detected that the reaction was complete. Water (50 mL) was added to the reaction solution, extracted with ethyl acetate (60 mL), the organic phase was washed with saturated brine (50 mL*2), dried over anhydrous sodium sulfate, filtered and concentrated to obtain 2,6-dimethyl- 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine (1.2 g, 95.3% yield).
[0307] MS m / z(ESI):234.1[M+H] + .
[0308] The second st...
Embodiment 2
[0330] Preparation of 4-(5-(ethylsulfonylamino)-2-((4-fluorophenyl)amino)phenyl)-2,6-lutidine by 1-oxidation
[0331]
[0332] Using 4-(2-fluoro-5-nitrophenyl)-2,6-lutidine as the starting material, replacing 2,4-difluorophenol with 4-fluoroaniline, the preparation steps refer to Example 1, 4-(5-(ethylsulfonylamino)-2-((4-fluorophenyl)amino)phenyl)-2,6-lutidine 1-oxidation (20.0mg, yield 24.5%) .
[0333] 1 H NMR (400MHz, CDCl 3 )δ:7.28(s,2H),7.38(m,3H),7.00(d,J=6.4Hz,4H),6.42(s,1H),5.52(s,1H),3.13(q,J=7.2 Hz, 2H), 2.55(s, 6H), 1.41(t, J=7.2Hz, 3H).
[0334] MS m / z(ESI):416.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com