Pharmaceutical composition containing mitochondria
A composition and mitochondrial technology, which can be used in drug combinations, medical preparations containing active ingredients, drug delivery, etc., and can solve problems such as adverse side effects, limited therapeutic scope, and no breakthrough therapeutic agents have yet been developed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0108] Hereinafter, preferred embodiments of the present invention will be described in order to facilitate understanding of the present invention. However, the following examples are provided only for easier understanding of the present invention, and the present invention is not limited by the following examples.
[0109] I. Confirmation of the effect of mitochondria and cells containing exogenous mitochondria in the treatment of muscle diseases
Embodiment 1
[0110] Example 1. Establishment of Muscle Atrophy Animal Model and Measurement of Body Weight Change
[0111] 5-week-old female SD rats as experimental animals were purchased from Oriental Bio Co., Ltd. (Seoul, Korea). Rats were acclimated in the clean area of the CHA University Laboratory Animal Center. During the acclimation period, the rats' environment was maintained on a 12-h light / dark cycle at room temperature of 23 ± 2 °C and humidity of 40 to 60%. After 7 days of such an acclimation period, experiments were performed on the rats.
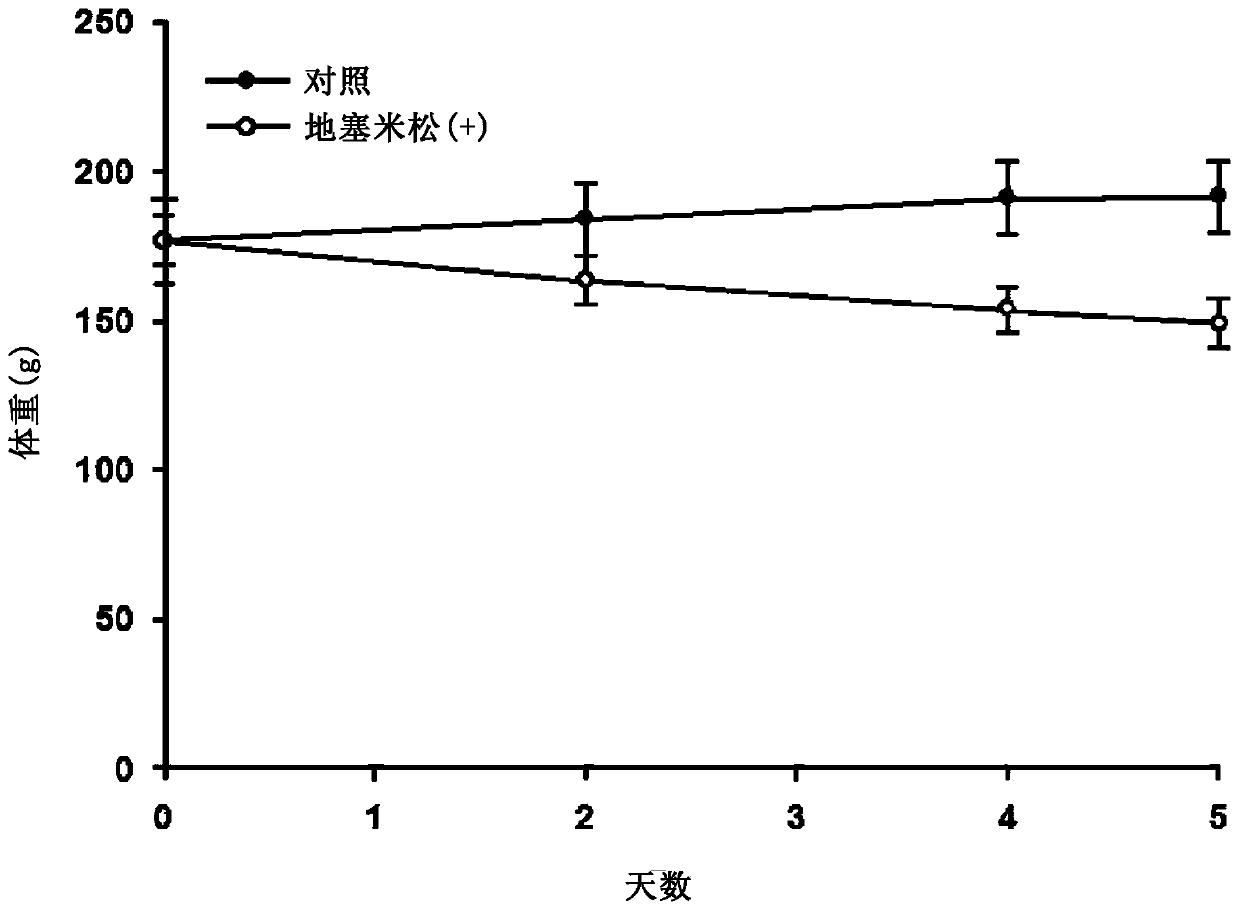
[0112] To induce muscle atrophy, dexamethasone was administered intraperitoneally to rats at a dose of 5 mg / kg for 5 days. The rats were weighed every day, and it was confirmed that the weight of the dexamethasone-treated group on the 5th day of dexamethasone administration decreased by about 30% compared with the normal group ( figure 1 ).
Embodiment 2
[0113] Example 2. Preparation of mitochondria for transplantation
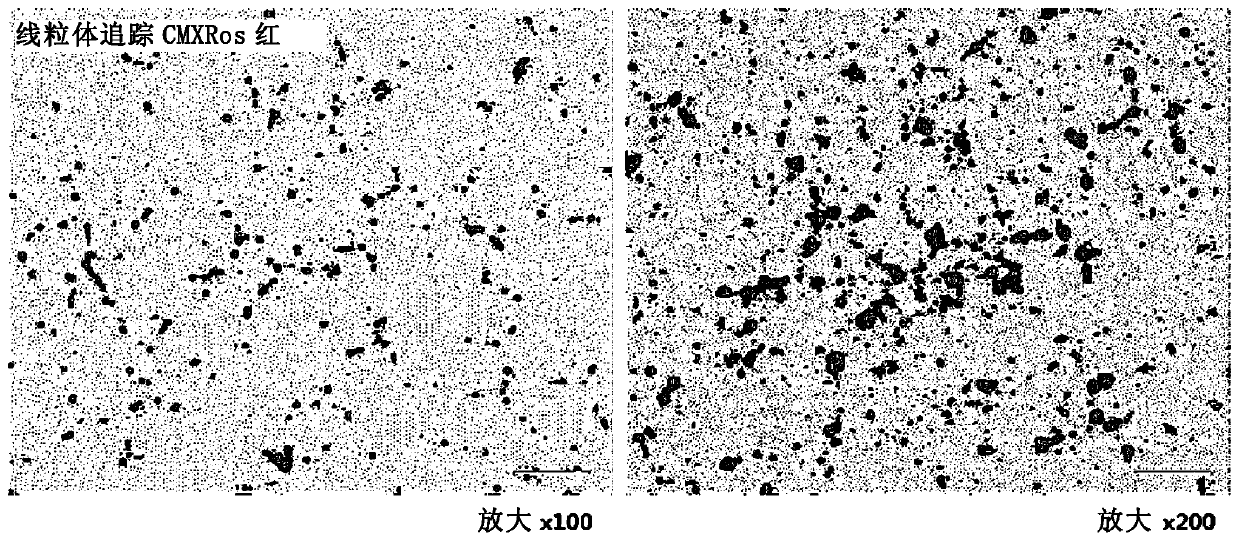
[0114] As donor cells for mitochondria, the rat skeletal muscle-derived cell line L6 (CRL1458, ATCC, Manassas, Virginia, USA) was cultured, and the resulting cells were stained with a mitochondria-specific marker (Mitotracker CMXRosRed) .
[0115] To extract mitochondria, measure the number of cells using a hemocytometer to recover approximately 2 × 10 7 cells / ml of cells. The cells were then subjected to a first centrifugation at 350 x g for 10 minutes at a temperature of about 4°C, and the resulting pellet was recovered and resuspended in buffer solution and then homogenized. The pharmaceutical composition containing the pellet was subjected to a second centrifugation at 1,100 xg for 3 minutes at a temperature of about 4°C to obtain a supernatant. The supernatant was then subjected to a third centrifugation at 12,000 xg for 15 minutes at about 4°C to isolate mitochondria from the cells.
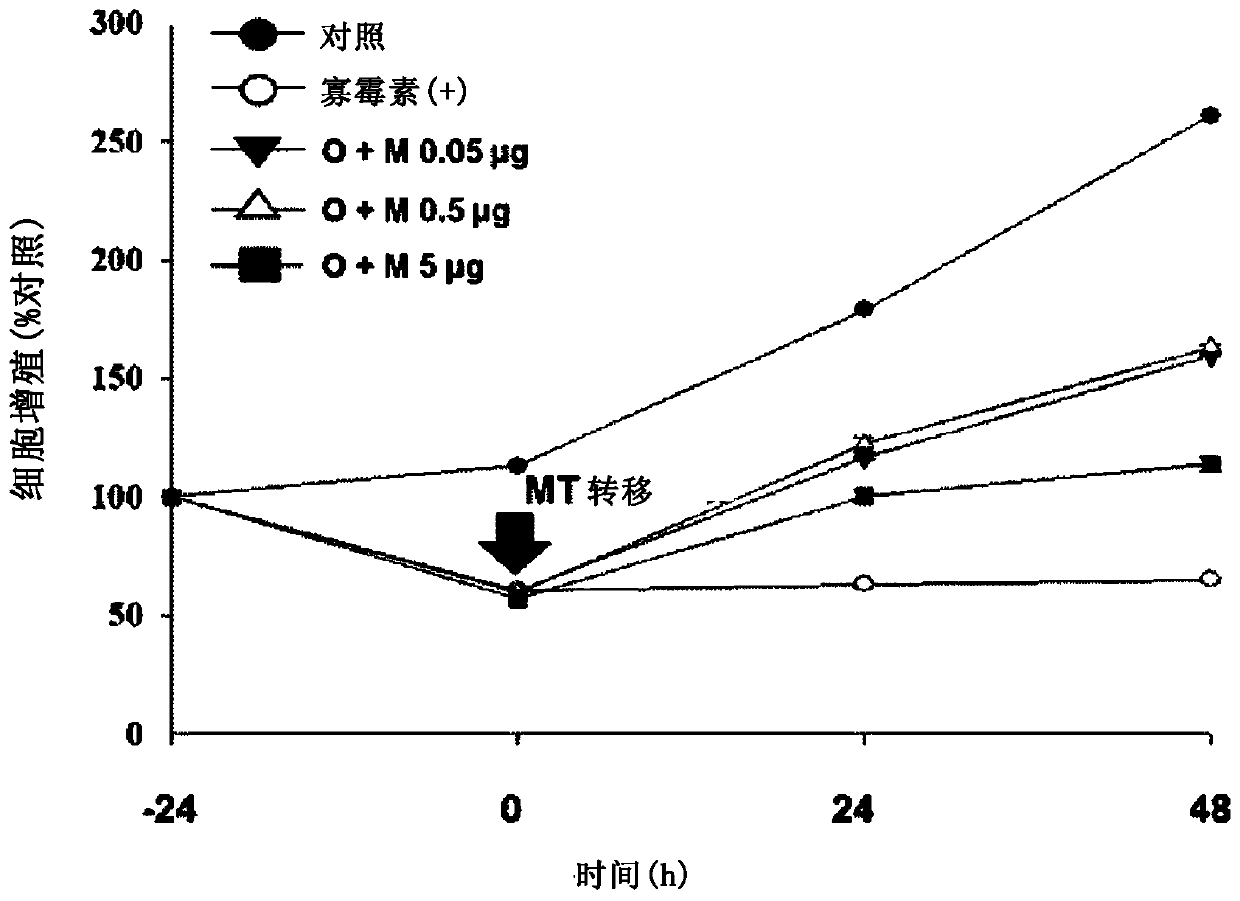
[0116] To ident...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com