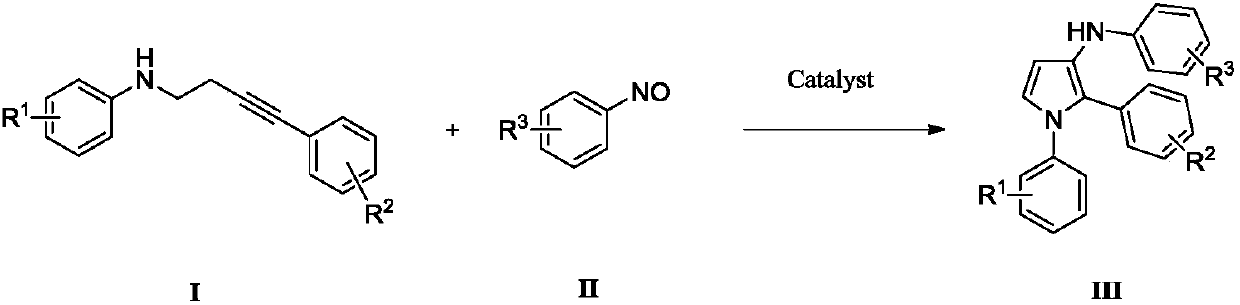
Novel preparation method for polysubstituted 3-aminopyrrole
An aminopyrrole, multi-substituted technology, applied in the direction of organic chemistry and the like, can solve the problems of complex reaction steps, high industrial cost, harsh synthesis reaction conditions, etc., and achieve the effects of simple preparation, environmental friendliness, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Taking the synthesis of N-1,2-triphenyl-1H-pyrrol-3-amine with the following structural formula as an example, the specific method includes the following steps:
[0051]
[0052]Add 1,4-dioxane (2mL), compound Ia (0.3mmol), nitrosobenzene IIa (0.6mmol), I 2 (0.03mmol), H 2 O (0.6mmol), and stirred and reacted at 90°C for 12h, and the reaction process was monitored by thin-layer chromatography until the reaction was complete; the mobile phase of petroleum ether / ethyl acetate=100 / 1 was separated and purified by silica gel column chromatography to obtain polysubstituted 3-aminopyrrole compound IIIaa, the recovery rate was 74%. The reaction equation is as follows:
[0053]
[0054] The structure, NMR, and high-resolution mass spectrometry data of the resulting product are as follows:
[0055] 1H NMR (400MHz, CDCl3, ppm): δ=7.29-7.25(m,2H),7.23-7.15(m,6H),7.12-7.08(m,4H),6.90-6.89(m,3H),6.78 -6.74(m,1H),6.42-6.41(d,J=4.0 Hz,1H),5.23(s,1H);13C NMR(100MHz,CDCl3,ppm):...
Embodiment 2
[0057] Taking the synthesis of N,1-diphenyl-2-(o-tolyl)-1H-pyrrol-3-amine with the following structural formula as an example, the specific method includes the following steps:
[0058]
[0059] Add 1,4-dioxane (2mL), compound Ib (0.3mmol), nitrosobenzene IIa (0.6mmol), I 2 (0.03mmol), H 2 O (0.6mmol), and stirred and reacted at 90°C for 12h, and the reaction process was monitored by thin-layer chromatography until the reaction was complete; the mobile phase of petroleum ether / ethyl acetate=100 / 1 was separated and purified by silica gel column chromatography to obtain polysubstituted 3-aminofuran compound IIIba, the recovery rate is 73%. The reaction equation is as follows:
[0060]
[0061] The structure, NMR, and high-resolution mass spectrometry data of the resulting product are as follows: 1 H NMR (400MHz, CDCl 3 , ppm): δ=7.24-7.15(m,6H),7.13-7.09(m,2H),7.03-6.98(m,2H),6.89-6.85(m,4H),6.78-6.74(m,1H ),6.41-6.40(d,J=4.0Hz,1H),5.23(s,1H),2.28(s,1H); 13 C NMR (10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com