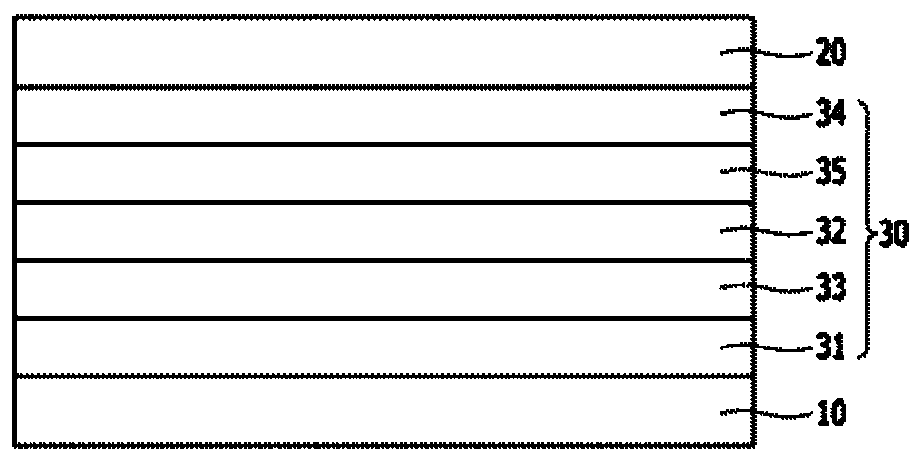
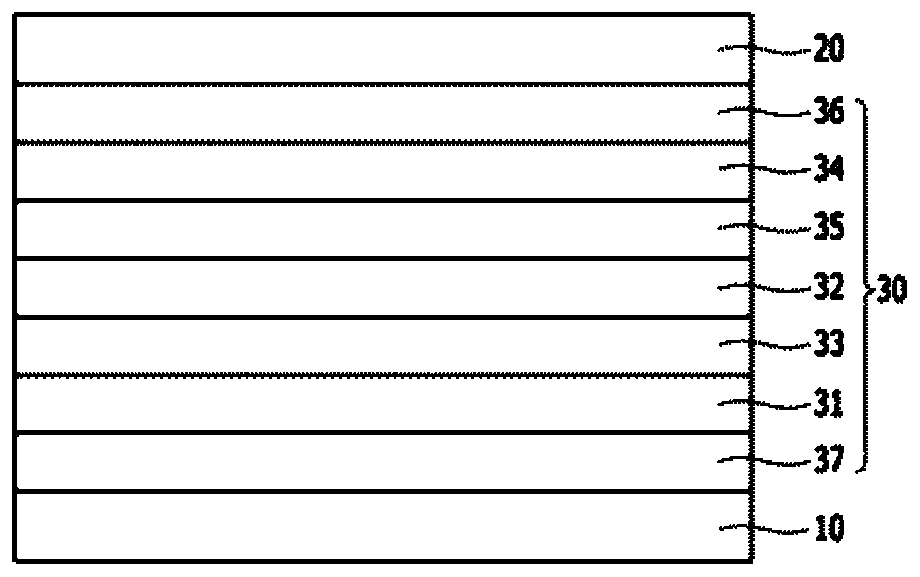
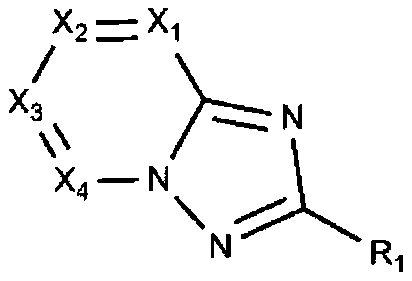
Organic compound and organic electroluminescent device comprising same
A compound and condensation technology, applied in the field of new organic compounds and organic electroluminescent elements, can solve the problems of poor thermal stability, unsatisfactory life of light-emitting elements, low glass transition temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1J-1
[0316] [Synthesis Example 1] Synthesis of J-1
[0317]
[0318] Under nitrogen flow, A-1 (2.48g, 10mmol), 2,4-diphenyl-6-(3-(4,4,5,5-tetramethyl-1,3,2-dioxa Cyclopentaboran-2-yl)phenyl)-1,3,5-triazine (4.35g, 10mmol), Pd(OAc) 2 (0.11g, 5mol%), Xphos (0.47g, 2mmol), Cs 2 CO 3 (6.51g, 20mmol), toluene / EtOH / H 2 O (80ml / 40ml / 20ml) was mixed and stirred at 110°C for 6 hours. After the reaction, extract with dichloromethane, add MgSO 4 to filter. The solvent of the filtered organic layer was removed, and then the target compound J-1 (2.57 g, yield 54%) was obtained by column chromatography.
[0319] [LCMS]: 476
Synthetic example 2J-2
[0320] [Synthesis Example 2] Synthesis of J-2
[0321]
[0322] Using A-2 (2.48g, 10.0mmol) instead of A-1, using 2,4-diphenyl-6-(3'-(4,4,5,5-tetramethyl-1,3,2- Dioxaborolan-2-yl)-[1,1'-biphenyl]-3-yl)-1,3,5-triazine (5.11g, 10.0mmol) instead of 2,4-bis Phenyl-6-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,5-tri Except for oxazine, the same procedure as in Synthesis Example 1 was carried out to obtain the target compound J-2 (3.03 g, yield 55%).
[0323] [LCMS]: 552
Synthetic example 3J-3
[0324] [Synthesis Example 3] Synthesis of J-3
[0325]
[0326] Using A-3 (2.48g, 10.0mmol) instead of A-1, using 10-phenyl-2'-(4,4,5,5-tetramethyl-1,3,2-dioxaborol Alk-2-yl)-10H-spiro[acridine-9,9'-fluorene] (5.33g, 10.0mmol) in place of 2,4-diphenyl-6-(3-(4,4,5,5 -Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,5-triazine, except that, implement the same process as Synthesis Example 1 to obtain Target compound J-3 (3.21 g, yield 56%).
[0327] [LCMS]: 574
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com