Capturing method and capturing kit for circulating tumor cells
A tumor cell and capture reagent technology, applied in the field of biomedicine, can solve problems such as insufficient sensitivity, low CTC content, lack of epidermal antigen expression capture, etc., to achieve the effect of improving capture efficiency and detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0029] According to a typical embodiment of the present invention, the capture method further includes: S4, detecting the number of captured cells by immunofluorescent staining; preferably, S4 specifically includes: S41, fixing the isolated circulating tumor cells and labeling the nuclei with DAPI fluorescent dye ; S42, cells were labeled with CD45 green fluorescent antibody; S43, cells were labeled with CKmix, EpCAM, vimentin red fluorescent antibody; S44, the results were analyzed under a three-color fluorescence microscope, DAPI+, CD45-, CKmix+ or EpCAM+ or vimentin+ cells Defined as CTCs.
[0030] According to a typical implementation of the present invention, the capture method includes:
[0031] 1. Isolation of Monocytes
[0032] 1) Collect 8 mL of peripheral blood from breast cancer patients with EDTA tubes.
[0033] 2) Dilute 8 mL of blood by 1 time with PBS.
[0034] 3) Add 15mL of Ficoll lymphocyte separation medium to a 50mL centrifuge tube, and carefully add the...
Embodiment 1
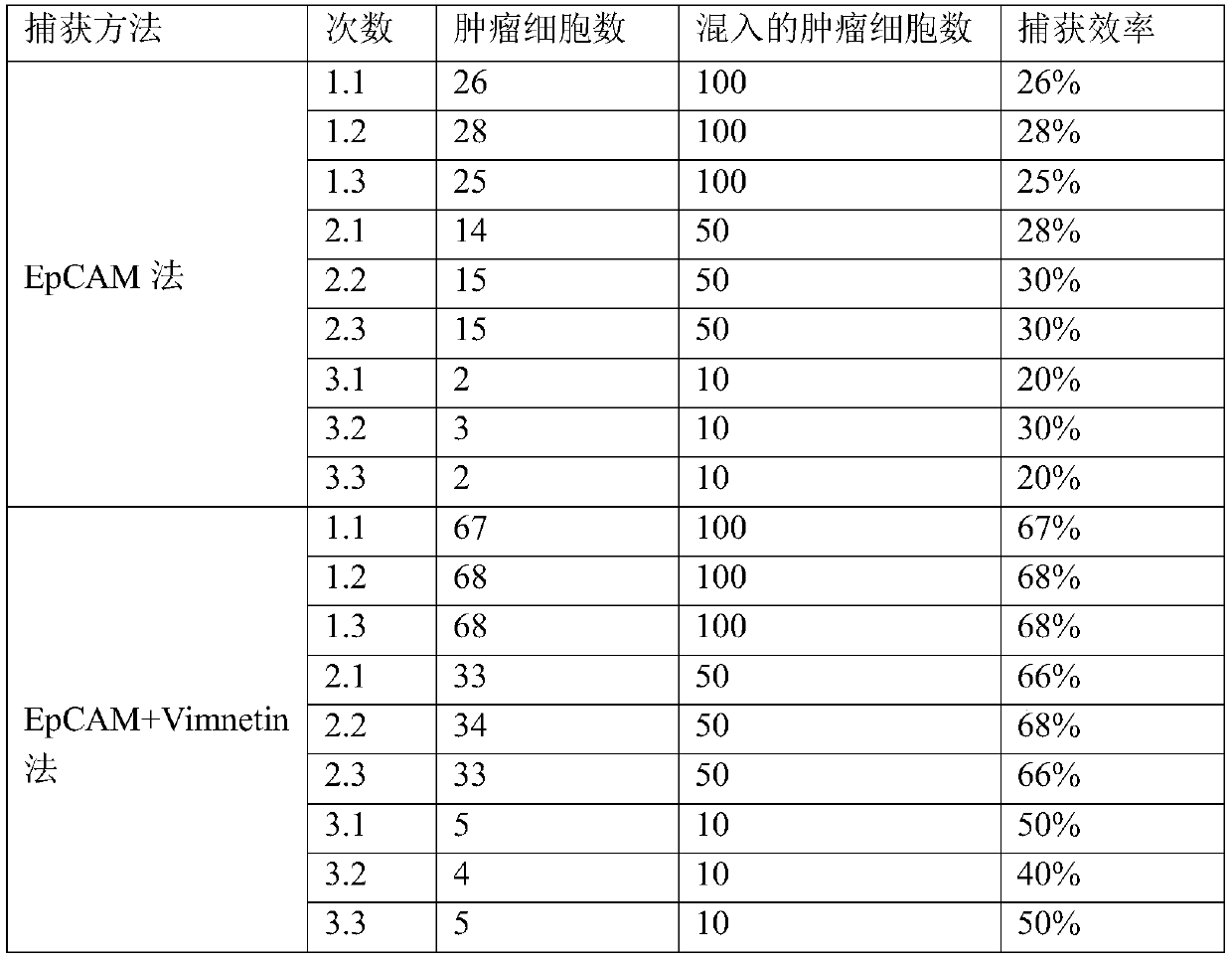
[0062] EpCAM alone captures peripheral blood CTCs and EpCAM+Vimentin captures peripheral blood CTCs capture efficiency verification.
[0063] Specific steps are as follows:
[0064] 1) Collect 8 mL of peripheral blood from a healthy person in an EDTA tube, and mix it with the same number of MCF-7 cells and MDA-MB-231 cells.
[0065] 2) Dilute one time with PBS.
[0066] 3) Add 15mL of Ficoll lymphocyte separation medium to a 50mL centrifuge tube, and carefully add the diluted blood to the upper layer of the separation medium.
[0067] 4) Centrifuge at 400g room temperature for 30min.
[0068] 5) If stratification occurs after centrifugation, take the buffy coat layer in the middle, which is the cell layer, and transfer it to a new 15mL centrifuge tube.
[0069] 6) Wash twice with PBS.
[0070] 7) Add 250 μL of CD45 magnetic beads, and incubate at 4° C. for 30 min on a rotary mixer.
[0071] 8) Stick the cell magnetic bead mixture on the magnetic stand.
[0072] 9) When t...
Embodiment 2
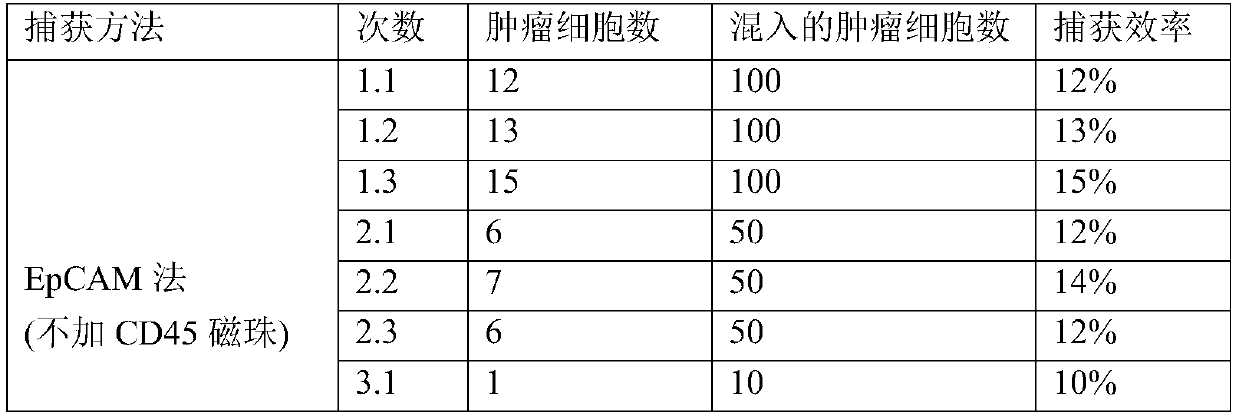
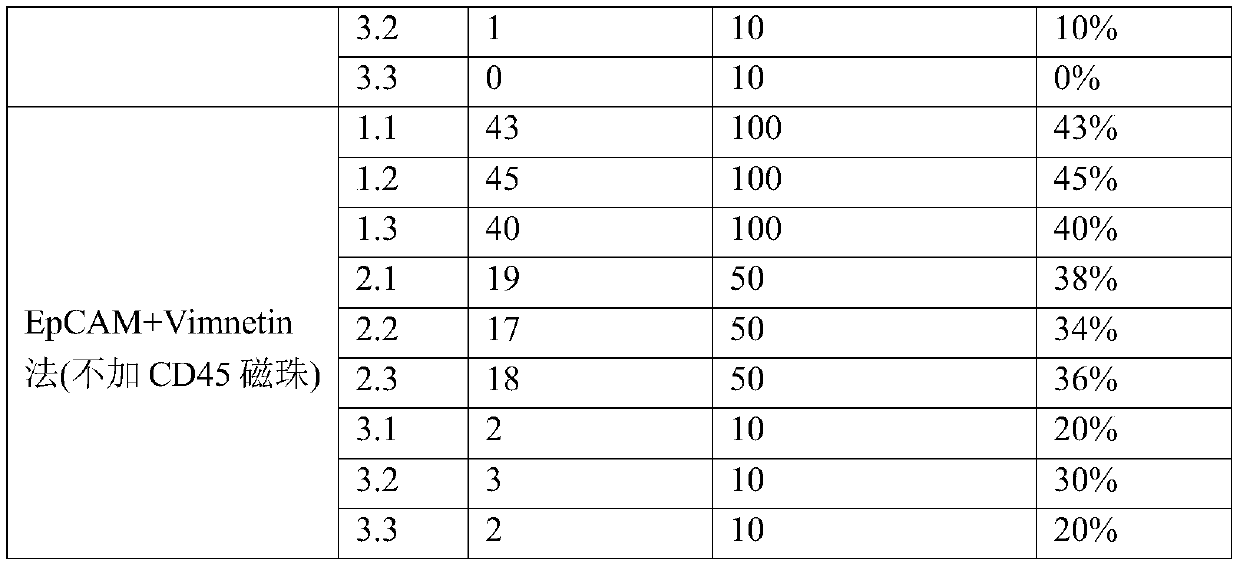
[0092] CD45 magnetic beads were not used to remove leukocytes, and EpCAM alone captured peripheral blood CTCs and EpCAM+Vimentin captured peripheral blood CTCs to verify the capture efficiency.
[0093] Specific steps are as follows:
[0094] 1) Collect 8 mL of peripheral blood from a healthy person in an EDTA tube, and mix it with the same number of MCF-7 cells and MDA-MB-231 cells.
[0095] 2) Dilute one time with PBS.
[0096] 3) Add 15mL of Ficoll lymphocyte separation medium to a 50mL centrifuge tube, and carefully add the diluted blood to the upper layer of the separation medium.
[0097] 4) Centrifuge at 400g room temperature for 30min.
[0098] 5) If stratification occurs after centrifugation, take the buffy coat layer in the middle, which is the cell layer, and transfer it to a new 15mL centrifuge tube.
[0099] 6) Wash twice with PBS.
[0100] 7) Only 1 μg EpCAM antibody was added to one group, and 1 μg EpCAM antibody and 1 μg Vimentin antibody were added to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com