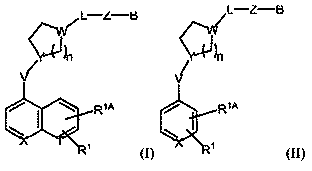
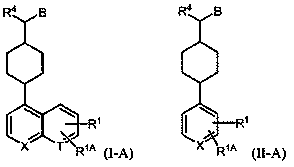
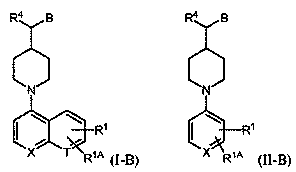
Inhibitors of indoleamine 2,3-dioxygenase and methods of their use
A C1-C6, OC1-C6 technology, applied in the field of enzyme active compounds, can solve problems such as different functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0060] In another embodiment, the present invention provides a combination comprising one or more compounds of the present invention and / or pharmaceutically acceptable salts thereof, stereoisomers thereof, tautomers thereof or solvates thereof things.
[0061] In another embodiment, the present invention provides a compound comprising a pharmaceutically acceptable carrier and at least one compound of the present invention and / or a pharmaceutically acceptable salt thereof, a stereoisomer thereof, a tautomer thereof or Pharmaceutical compositions of its solvates.
[0062] In another embodiment, the present invention provides a pharmaceutical composition comprising a pharmaceutically acceptable carrier and a therapeutically effective amount of at least one compound of the present invention and / or pharmaceutically acceptable salts, stereoisomers thereof body, its tautomer or its solvate.
[0063] In another embodiment, the present invention provides a process for the preparation...
Embodiment 1
[0242] 4-(( cis )-4-((R)-1-(1H-benzo[d]imidazol-2-yl)ethyl)cyclohexyl)-6-fluoroquinoline
[0243]
[0244] Embodiment 1: 4-(( cis )-4-((R)-1-(1H-benzo[d]imidazol-2-yl)ethyl)cyclohexyl)-6-fluoroquinoline
[0245] Preparation 1A:
[0246]
[0247] in N 2 1,4-dioxaspiro[4.5]decane-8-one (300 g, 1920.86 mmol, 1.0eq) and N To a stirred solution of -phenyltrifluoromethanesulfonimide (823.47 g, 2305.03 mmol, 1.2eq) in MTBE (7.5 L) was added 2.0 M NaHMDS / THF (1152.2 mL, 2305.03 mmol, 1.2eq), and The mixture was stirred for another 60 minutes. The reaction mixture was warmed to room temperature and stirred overnight until TLC showed complete consumption of starting material. The mixture was treated with KHSO 4 Aqueous solution (100 ml) was quenched, filtered to remove solids and the filtrate was concentrated completely. 3 LMTBE was added to the residue, followed by washing with 5% NaOH (1.5 L×3). The organic phase was concentrated to obtain 567 g of crude Preparation 1...
Embodiment 2-7
[0285]
[0286] Examples 2-7 were prepared from Preparation 1K using the procedure of Example 1 and the corresponding phenylenediamines.
[0287] Example number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com