Whole-course acquisition method of dissolved inorganic carbon content and carbon isotope value of environmental water body
A carbon isotope and acquisition method technology, applied in inorganic chemistry, chemical instruments and methods, water pollutants, etc., can solve the problems of lack of verification work, easy needle blocking, poor operability of anhydrous solid phosphoric acid, etc., to achieve flexible and convenient operation, Guaranteed authentic reproduction of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
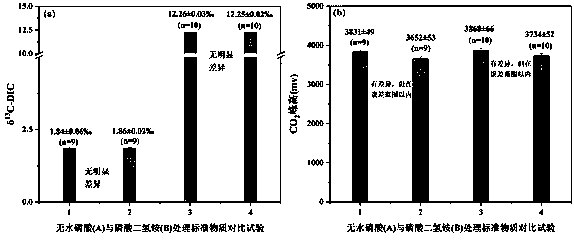
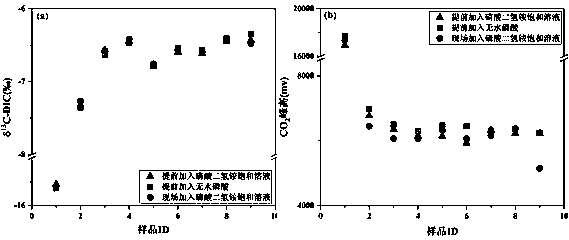
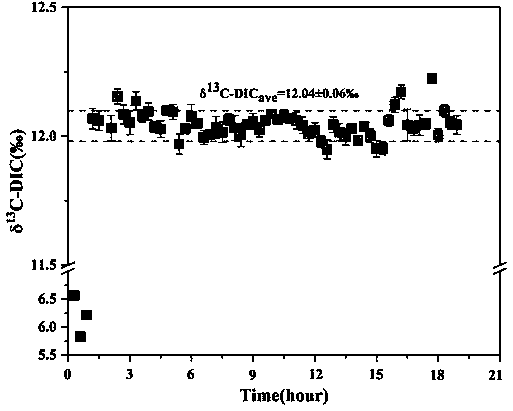
[0086] In December 2018, the water samples of Yihe River were collected, and the saturated ammonium dihydrogen phosphate solution was used on site to directly react with the collected and filtered water samples to produce CO 2 , producing CO 2 Store in headspace vials at room temperature. After 30 days, the laboratory randomly selected 5 samples, and used saturated ammonium dihydrogen phosphate solution to react with the water samples backed up in the field again to produce CO 2 , tested together with the samples collected before, the results are as follows Figure 5 shown.
[0087] From Figure 5 It can be seen that whether the water sample is directly treated with saturated ammonium dihydrogen phosphate solution in the field, or the water sample is treated with saturated ammonium dihydrogen phosphate solution after transporting the sample, there is a good linear relationship between the two, which shows that this method Good stability. However, the results show that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com