Chenodeoxycholic acid and preparation method thereof
A technology of chenodeoxycholic acid and cholic acid, which is applied in the field of drug synthesis, can solve the problems of large environmental impact, and achieve the effects of mild conditions, high yield and strong reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
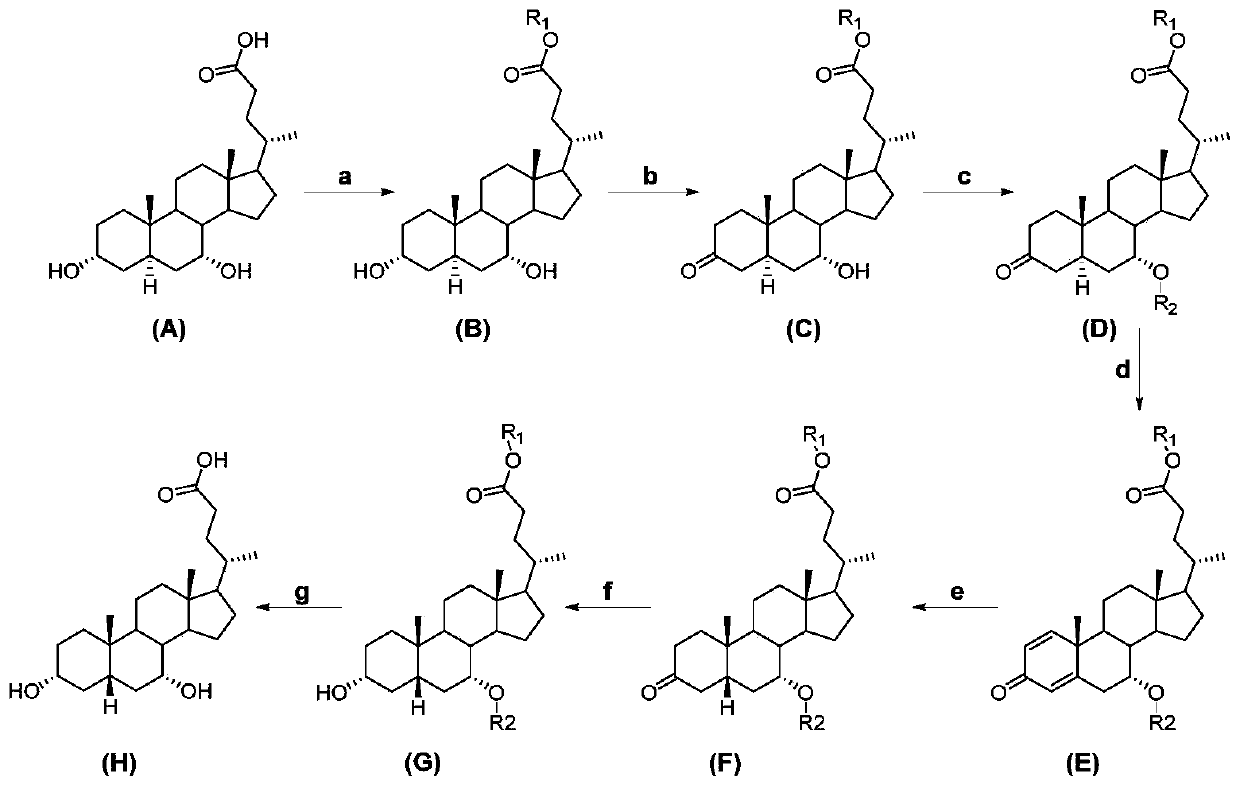
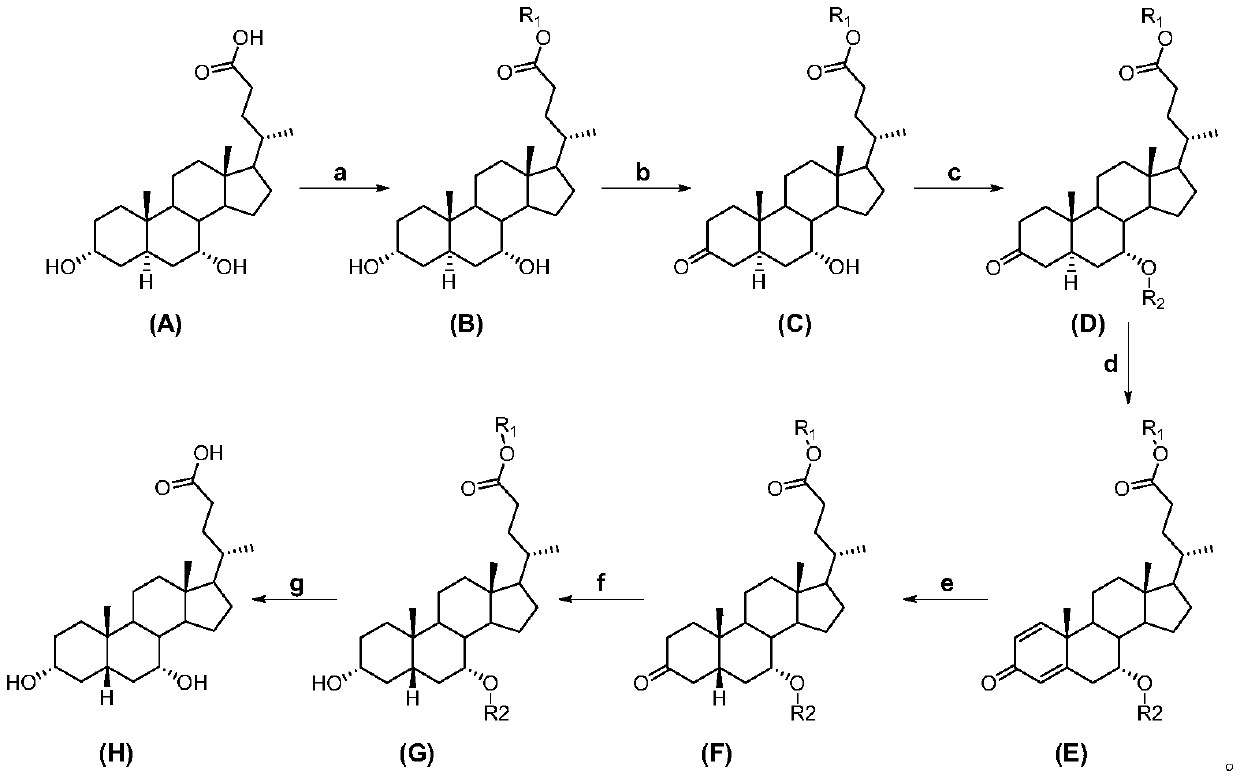
[0015] A kind of preparation method of chenodeoxycholic acid, see figure 1 , including the following steps:
[0016] Carry out the chemical reaction according to the following formula:
[0017]
[0018] In the embodiment of the present invention, 3α, 7α-dihydroxy-5α-cholanic acid (abbreviation: allochodeoxycholic acid) (A) is used as raw material to obtain 3α, 7α-dihydroxy-5α-cholanic acid ester ( B), 3α,7α-dihydroxy-5α-cholanate (B) 3-position selective oxidation to 3-keto-7α-hydroxy-5α-cholanate (C), 3-keto-7α-hydroxy -Protection of the 7-position of 5α-acyloxy-5α-cholanate (C) gives 3-keto-7α-acyloxy-5α-cholanate (D), 3-keto-7α-acyloxy-5α-cholanic acid Ester (D) undergoes an oxidation reaction to obtain △1,4-3-keto-7α-acyloxy-unsaturated cholanoic acid ester (E), △1,4-3-keto-7α-acyloxy-unsaturated Cholanate (E) is subjected to catalytic hydrogenation to reduce the double bond to obtain 3-keto-7α-acyloxy-5β-cholanate (F), 3-keto-7α-acyloxy-5β-cholanate The 3-position ...
Embodiment 1
[0063] This embodiment provides a method for preparing chenodeoxycholic acid, comprising the following steps:
[0064] S1, preparation of intermediate B;
[0065] Add allchenodeoxycholic acid (1.0 g, 2.6 mmol), 15 mL of anhydrous methanol, and 100 μL of concentrated sulfuric acid into a reaction vessel equipped with a dry reflux condenser. After the addition, the reaction temperature is raised to 67° C., and the reaction is stirred for 4 h. After the reaction was completed, the solvent methanol was removed by rotary evaporation, and 20 mL of ethyl acetate was added to dissolve the residue, followed by 10 mL of saturated NaHCO 3 solution and water wash. The organic phase was washed with anhydrous MgSO 4 Dry to remove water, filter to remove anhydrous MgSO 4 , collected the organic phase, and distilled off the solvent under reduced pressure to obtain 1.0 g of white solid, which was intermediate B, yield: 99%.
[0066] The structural characterization data of intermediate B ar...
Embodiment 2- Embodiment 10
[0108] The preparation method of chenodeoxycholic acid provided in Example 2-Example 10 is basically similar to the operation of the preparation method of chenodeoxycholic acid provided in Example 1, except that the raw materials used and the specific conditions of each step are different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com