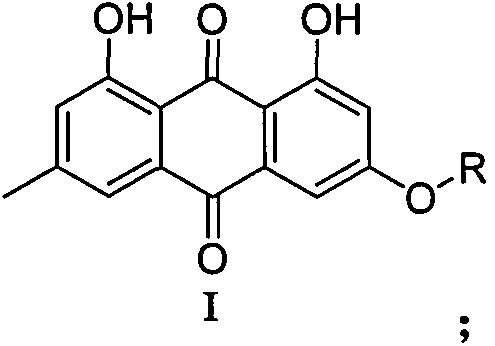
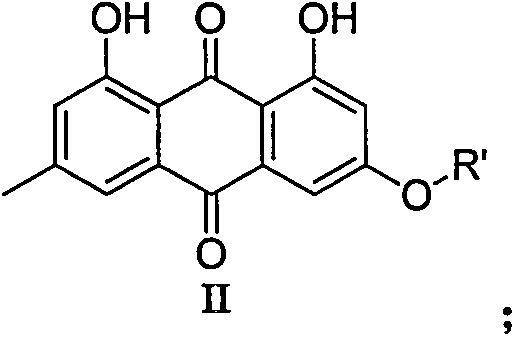
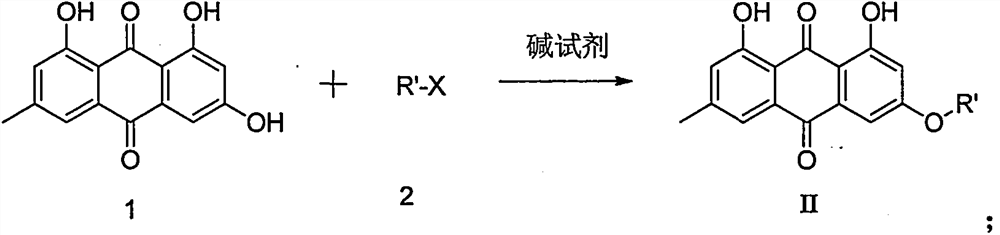
9,10-anthraquinone derivatives, their preparation methods and applications
An anthraquinone derivative and drug technology, applied in 9 fields, can solve the problems of general action strength, increase activity, etc., and achieve the effect of good anti-HCV activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Dissolve 5mmol emodin in 15mL acetone, add 10mmol potassium carbonate and 15mmol chloroacetonitrile, heat up to 45°C for 12 hours, distill off the solvent under reduced pressure, add 15mL water, then extract three times with 15mL ethyl acetate, combine the organic phases, 15mL After washing once with water, the compound 1 was obtained by column chromatography (PE / EA=4 / 1) with a yield of 59%.
[0039] 1 H NMR (500MHz, CDCl 3 )δ12.29(s, 1H), 11.99(s, 1H), 7.65(s, 1H), 7.41(d, J=2.5Hz, 1H), 7.11(s, 1H), 6.79(d, J=2.0 Hz, 1H), 4.91(s, 2H), 2.47(s, 3H); 13 C NMR (126MHz, CDCl 3 )δ191.1, 181.4, 164.9, 162.7, 162.6, 149.1, 135.8, 132.9, 124.8, 121.6, 113.8, 113.5, 111.9, 108.1, 107.1, 53.3, 22.2; ESI-MS: m / z 310.2 [M+H] + ;307.9[M-H] - .
Embodiment 2
[0041] The reaction conditions and operations were the same as in Example 1, except that chloroacetonitrile was replaced by ethyl bromoacetate to obtain compound 2 with a yield of 45%.
[0042] 1 H NMR (500MHz, CDCl 3 )δ12.27(s, 1H), 12.05(s, 1H), 7.62(s, 1H), 7.37(d, J=2.5Hz, 1H), 7.09(s, 1H), 6.68(d, J=2.5 Hz, 1H), 4.75(s, 2H), 4.29(q, J=2.0Hz, 2H), 2.45(s, 3H), 1.32(t, J=7.2Hz, 3H); 13 C NMR (126MHz, CDCl 3 )δ190.9, 181.7, 167.5, 164.9, 164.5, 162.6, 148.7, 135.4, 133.1, 124.6, 121.4, 113.6, 111.0, 107.9, 107.8, 65.2, 61.8, 29.7, 22.1, 14.1; ESI-MS: m / z 355.0[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com