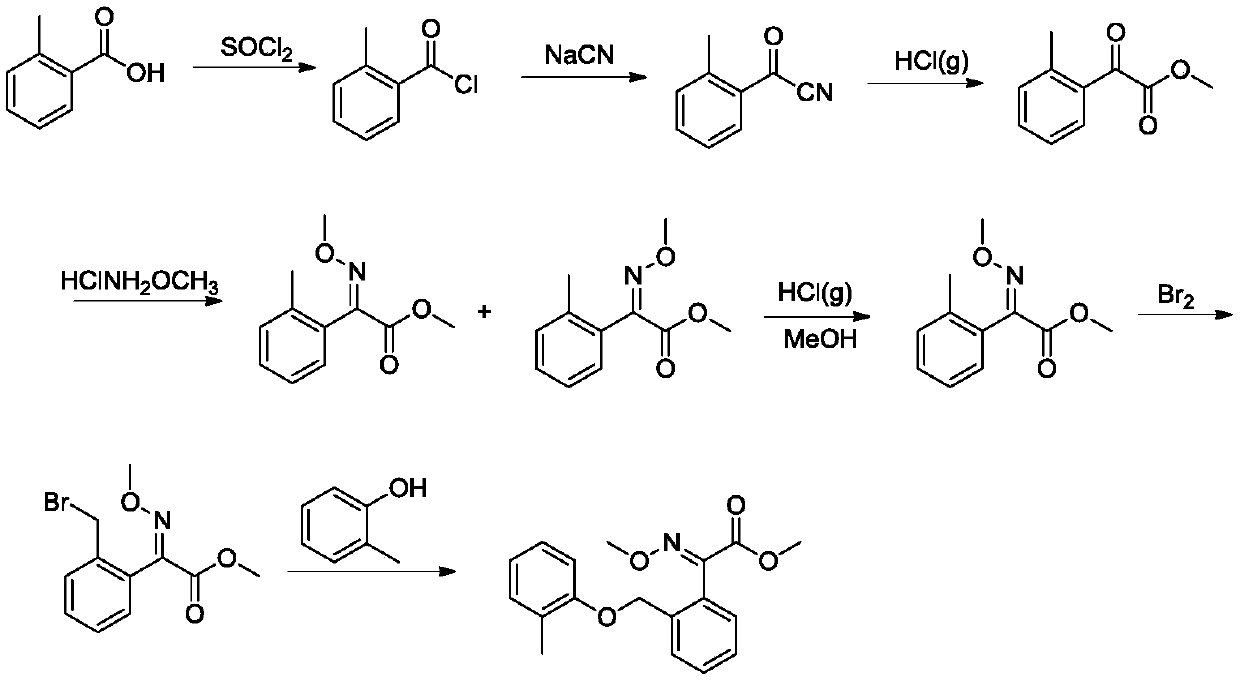
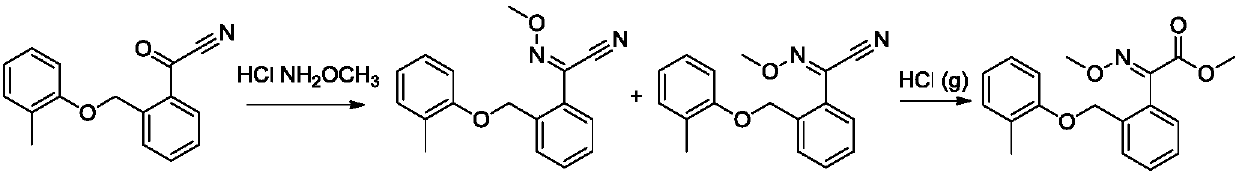
Synthetic method of kresoxim methyl
A synthetic method, the technology of kresoxim-methyl, which is applied in the field of fine chemical industry, can solve the problems of high cost and achieve the effects of low cost, improved process yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of synthetic method of kresoxim-methyl, its specific steps are:
[0030] (1) Preparation of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile
[0031] Add 1mol of 2-(2-methylphenoxymethyl)benzoyl cyanide and 1.1mol of methoxylamine hydrochloride to 5 times the volume of methanol, drop into 0.01mol of benzyltriethylammonium chloride, and heat up To 60°C, keep warm until 2-(2-methylphenoxymethyl)benzoyl cyanide remains 0.5%, to obtain 2-methoxyimino-2-[2-(2-methylphenoxymethyl) ) methanol solution of phenyl] acetonitrile;
[0032] (2) Preparation of Kresoxim-methyl by pinner reaction
[0033] After the methanol solution of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile obtained above was cooled to 5°C, hydrochloric acid gas was passed into the system, and the amount of hydrochloric acid gas It is 1.1mol. After the hydrochloric acid gas is passed through, the temperature is slowly raised to 15°C, and the reaction is kept until the rem...
Embodiment 2
[0035] A kind of synthetic method of kresoxim-methyl, its specific steps are:
[0036] (1) Preparation of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile 2-(2-methylphenoxymethyl)benzoyl Add cyanogen and 2.1mol methoxyamine hydrochloride to 6 times the volume of methanol, add 0.02mol tetrabutylammonium bromide, heat up to 58°C, and keep warm until 2-(2-methylphenoxymethyl)benzyl Acyl cyanide remained 0.5% to obtain a methanol solution of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile;
[0037] (2) Preparation of Kresoxim-methyl by pinner reaction
[0038] After the methanol solution of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile obtained above was cooled to 8°C, hydrochloric acid gas was passed into the system, and the amount of hydrochloric acid gas It is 2.3mol. After the hydrochloric acid gas is passed through, the temperature is slowly raised to 20°C, and the reaction is kept until 2-methoxyimino-2-[2-(2-methylphenoxymeth...
Embodiment 3
[0040] A kind of synthetic method of kresoxim-methyl, its specific steps are:
[0041] (1) Preparation of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile with 2.5mol of 2-(2-methylphenoxymethyl)benzene Acyl cyanide and 2.7mol methoxylamine hydrochloride were added to 3 times the volume of methanol, 2.7mol of 4-dimethylaminopyridine was added, the temperature was raised to 55°C, and the temperature was kept until 2-(2-methylphenoxymethyl)benzene 0.5% of formyl cyanide remained to obtain a methanol solution of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile.
[0042] (2) Preparation of Kresoxim-methyl by pinner reaction
[0043] After the methanol solution of 2-methoxyimino-2-[2-(2-methylphenoxymethyl)phenyl]acetonitrile obtained above was cooled to 10°C, hydrochloric acid gas was passed into the system, and the amount of hydrochloric acid gas It is 3.1mol, after the hydrochloric acid gas is passed through, the temperature is slowly raised to 18°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com