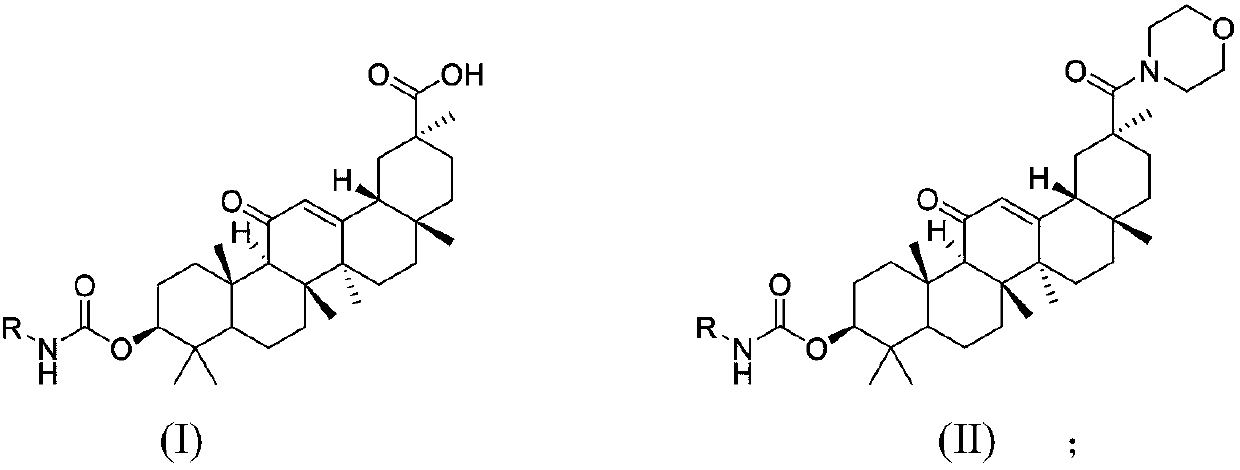
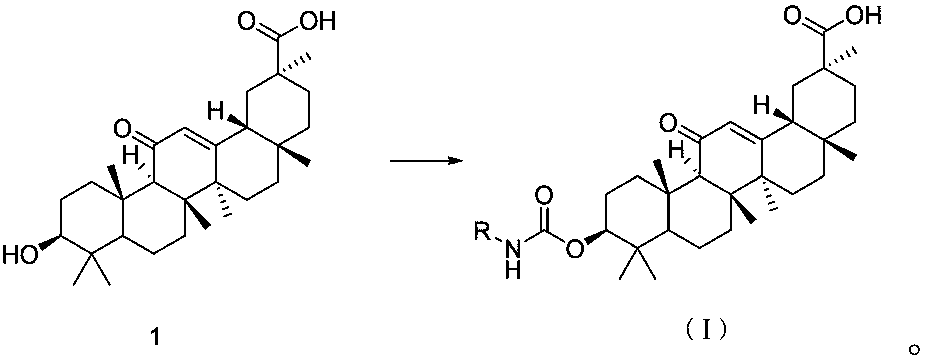
18beta-glycyrrhetinic acid carbamate derivative, preparation method and application thereof
An acid carbamate, glycyrrhetic acid technology, applied in the direction of drug combinations, steroids, antitumor drugs, etc., can solve the problem of unsatisfactory pharmacological activity, and achieve a new effect of compound structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of 3β-(((3,4-dichlorophenyl)carbamoyl)oxy group)-11-oxo-olean-12-ene-29-acid (abbreviation: Ia) shown in the following structural formula , including the following steps:
[0044]
[0045] 18β-glycyrrhetinic acid (0.30mmol) and 3,4-dichlorophenylisocyanate (0.36mmol) were added to reflux reaction in ethyl acetate; after the reaction was completed, the mixture was concentrated under reduced pressure, and the resulting residue was passed through a column Purified by chromatography to obtain a white powdery solid with a yield of 78.1%; 1 H NMR(400MHz,Chloroform-d)δ7.63(s,1H,phenyl-H),7.33(d,J=8.7Hz,1H,phenyl-H),7.18(d,J=8.8Hz,1H,phenyl -H),6.71(s, 1H,N-H),5.70(s,1H,CH-12),4.50(dd,J=10.5,5.9Hz,1H,CH-3),2.87–2.77(m,1H, CH-1), 2.37(s,1H,CH-9), 2.18(dd,J=13.8,4.1Hz,1H,CH-16),2.06–1.00(m,17H), 1.37(s,3H,CH 3 -27),1.22(s,3H,CH 3 -25),1.16(s,3H,CH 3 -26),1.12(s,3H,CH 3 -29), 0.93(s,3H,CH 3 -23),0.87(s,3H,CH 3 -24),0.82(s,3H,CH 3 -28),0.80(d,1H,C...
Embodiment 2
[0047] 3β-(((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)oxy)-11-oxo-olean-12-en-29-acid (referred to as : the preparation method of Ib), comprising the steps of:
[0048]
[0049] 18β-glycyrrhetinic acid (0.30mmol) and 4-chloro-3-(trifluoromethyl)phenylisocyanate (0.36mmol) were added to reflux reaction in dichloromethane; after the reaction was over, the mixture was concentrated under reduced pressure, and The obtained residue was purified by column chromatography; a white powdery solid was obtained with a yield of 87.0%; 1 H NMR (400MHz, Chloroform-d) δ 7.76(s,1H,phenyl-H),7.54(s,1H,phenyl-H),7.40(d,J=8.7Hz,1H,phenyl-H),6.83( s, 1H, N-H), 5.70(s, 1H, CH-12), 4.56–4.47(m, 1H, CH-3), 2.82(d, J=13.6Hz, 1H, CH-1), 2.37(s ,1H,CH-9),2.17(d,J=11.9Hz,1H,CH-16),2.06–1.00(m,17H),1.37(s,3H,CH 3 -27),1.22(s,3H,CH 3 -25),1.16(s,3H,CH 3 -26),1.12(s,3H,CH 3 -29),0.94(s,3H,CH 3 -23),0.88(s,3H,CH 3 -24),0.82(s,3H,CH 3 -28),0.80(d,1H,CH-5); HRMS(m / z):[M +H] + calcd.ForC 38 h ...
Embodiment 3
[0051] The preparation method of 3β-(((3,5-dichlorophenyl)carbamoyl)oxy)-11-oxo-olean-12-ene-29-acid (abbreviation: Ic) as shown in the following structural formula , including the following steps:
[0052]
[0053] 18β-glycyrrhetinic acid (0.30mmol) and 3,5-dichlorophenylisocyanate (0.36mmol) were added to reflux reaction in chloroform; after the reaction was completed, the mixture was concentrated under reduced pressure, and the resulting residue was passed through a column Purified by chromatography to obtain a white powdery solid with a yield of 78.1%; 1 H NMR (400MHz, Chloroform-d) δ7.35(s, 2H, phenyl-H), 7.02(t, J=1.8Hz, 1H, phenyl-H), 6.62(s, 1H, N-H), 5.69(s ,1H,CH-12),4.50 (dd,J=10.7,5.7Hz,1H,CH-3),2.82(d,J=13.2Hz,1H,CH-1),2.37(s,1H,CH- 9), 2.17 (d, J=11.9Hz, 1H, CH-16), 2.03–0.83 (m, 17H), 1.37 (s, 3H, CH 3 -27),1.22(s,3H,CH 3 -25),1.16(s,3H,CH 3 -26),1.12(s,3H,CH 3 -29),0.94(s,3H,CH 3 -23),0.88(s,3H,CH 3 -24),0.82(s,3H,CH 3 -28),0.79–0.73(m,1H,CH-5); HRM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com