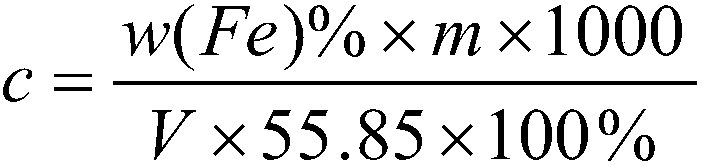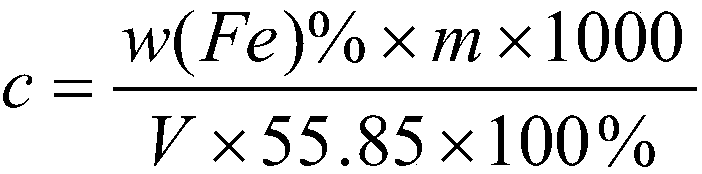Calibration method of potassium dichromate standard solution
A standard solution, potassium dichromate technology, applied in chemical method analysis, chemical analysis using titration method, measuring device and other directions, can solve the problems of uncertain concentration of potassium dichromate standard solution, high preparation cost, etc. Master and calibrate accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
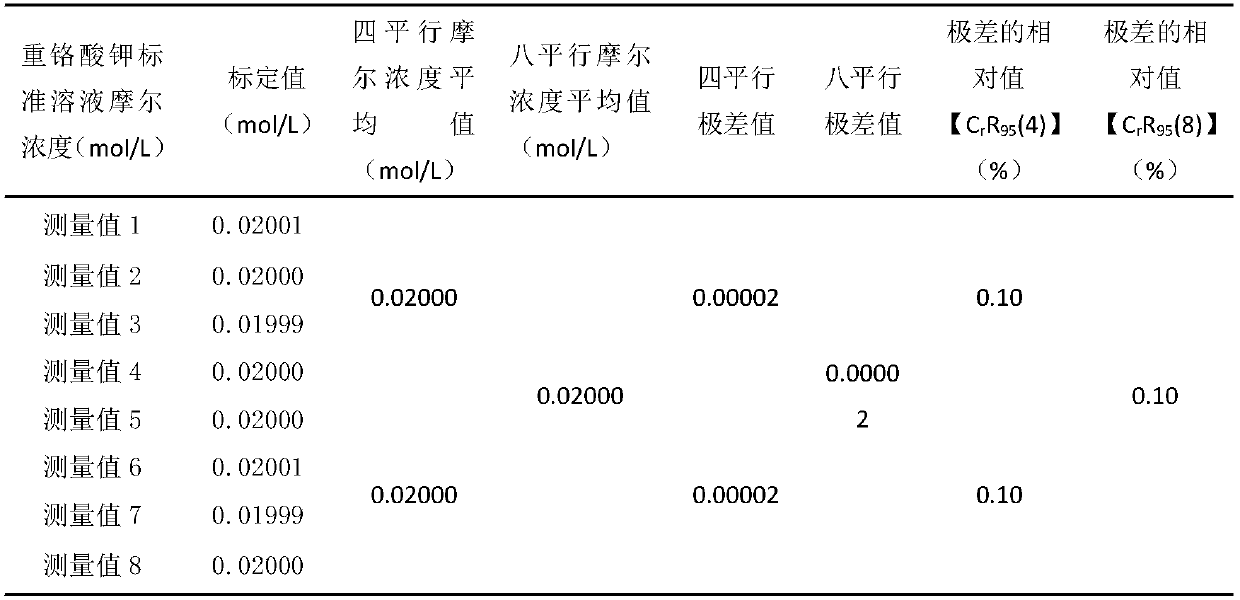
[0047] Embodiment one: calibration potassium dichromate standard solution molar concentration 0.02000mol / L:
[0048] 1. Weigh 0.04000±0.0001g of pure iron (content above 99.95%) into a 500mL Erlenmeyer flask, slowly add 20-30mL of concentrated hydrochloric acid, and dropwise add 1mL of concentrated nitric acid;
[0049] 2. Boil at low temperature on a 300°C electric heating plate to remove nitrogen oxides. During the dissolution process, continuously add 6% stannous chloride solution and a certain amount of 1:1 hydrochloric acid to keep the solution slightly yellow, and the concentrated volume is about 20mL;
[0050] 3. The sample is completely dissolved and there are big bubbles, take it out and cool it down slightly;
[0051] 4. Add about 100mL of water, 1mL of 25% sodium tungstate solution, and dropwise add titanium trichloride solution 1:19 under constant shaking until the solution turns blue;
[0052] 5. Add the diluted potassium dichromate standard solution dropwise unt...
Embodiment 2
[0061] Embodiment two: calibration potassium dichromate standard solution molar concentration 0.05000mol / L:
[0062] 1. Weigh 0.1000±0.0001g of pure iron (content above 99.95%) into a 500mL Erlenmeyer flask, slowly add 20-30mL of concentrated hydrochloric acid, and dropwise add 1mL of concentrated nitric acid;
[0063] 2. Boil at low temperature on a 300°C electric heating plate to remove nitrogen oxides. During the dissolution process, continuously add 6% stannous chloride solution and a certain amount of 1:1 hydrochloric acid to keep the solution slightly yellow, and the concentrated volume is about 20mL;
[0064] 3. The sample is completely dissolved and there are big bubbles, take it out and cool it down slightly;
[0065] 4. Add about 100mL of water, 1mL of 25% sodium tungstate solution, and dropwise add titanium trichloride solution 1:19 under constant shaking until the solution turns blue;
[0066] 5. Add the diluted potassium dichromate standard solution dropwise unti...
Embodiment 3
[0074] Embodiment three: calibration potassium dichromate standard solution molar concentration 0.1000mol / L:
[0075] 1. Weigh 0.2000±0.0001g of pure iron (content above 99.95%) into a 500mL Erlenmeyer flask, slowly add 20-30mL of concentrated hydrochloric acid, and dropwise add 1mL of concentrated nitric acid;
[0076] 2. Boil at low temperature on a 300°C electric heating plate to remove nitrogen oxides. During the dissolution process, continuously add 6% stannous chloride solution and a certain amount of 1:1 hydrochloric acid to keep the solution slightly yellow, and the concentrated volume is about 20mL;
[0077] 3. The sample is completely dissolved and there are big bubbles, take it out and cool it down slightly;
[0078] 4. Add about 100mL of water, 1mL of 25% sodium tungstate solution, and dropwise add titanium trichloride solution 1:19 under constant shaking until the solution turns blue;
[0079] 5. Add the diluted potassium dichromate standard solution dropwise unt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com