Method for determining silodosin impurities by virtue of HPLC process
A technology for silodosin impurities and silodosin, which is applied in the field of silodosin determination, can solve the problems of unstable nature, easy degradation, and unavailability of silodosin impurity detection, and achieves high sensitivity and exclusive use. The effect of good performance and short analysis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
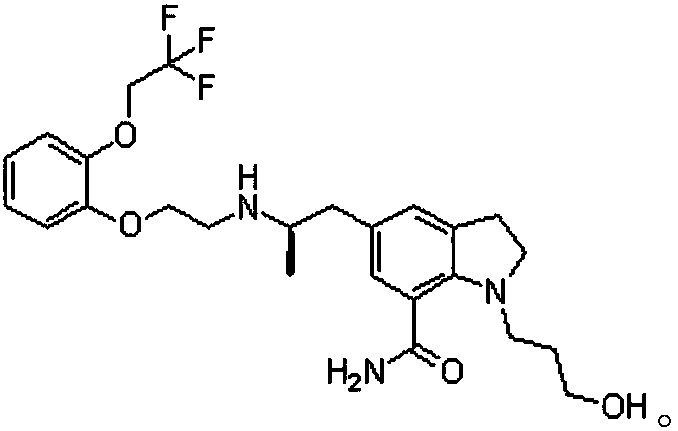
[0038] A method for HPLC method to measure silodosin impurity, described method comprises the steps:
[0039] S1. Preparation of system suitability solution: take a silodosin sample, expose to light, add methanol to the irradiated silodosin sample, and shake well to obtain a system suitability solution;
[0040] S2, preparation of reference substance solution: add methanol to the silodosin reference substance, shake well to obtain the reference substance solution;
[0041] S3, preparing the test product solution: add methanol to the silodosin test product, shake up to obtain the test product solution;
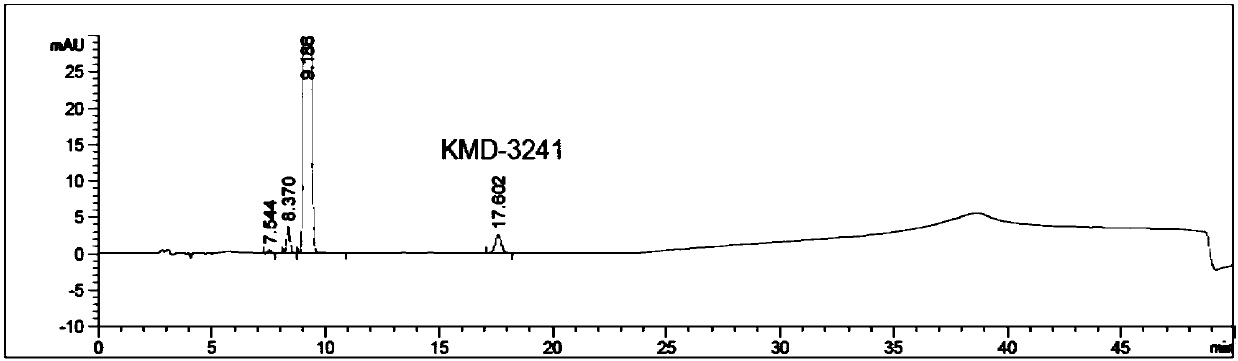
[0042] S4. Chromatographic determination: take the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram Figure I Get reference substance solution, inject liquid chromatograph, adjust detection sensitivity; Get need testing solution and reference substance solution, inject liquid chromatograph respectively, record chromatogram Figu...
Embodiment 2
[0044] A method for HPLC method to measure silodosin impurity, described method comprises the steps:
[0045] S1. Preparation of system suitability solution: take a silodosin sample, expose to light, add methanol to the irradiated silodosin sample, and shake well to obtain a system suitability solution;
[0046] S2, preparation of reference substance solution: add methanol to the silodosin reference substance, shake well to obtain the reference substance solution;
[0047] S3, preparing the test product solution: add methanol to the silodosin test product, shake up to obtain the test product solution;
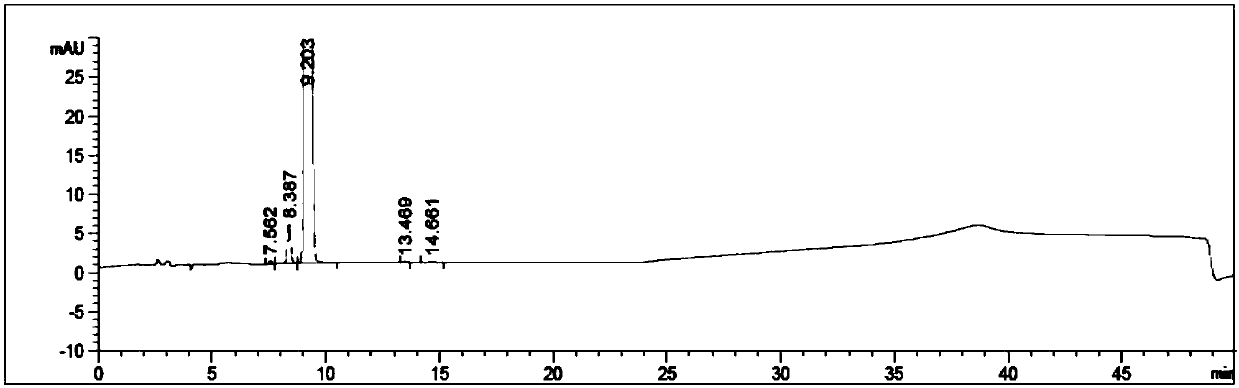
[0048] S4. Chromatographic determination: take the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram Figure I Get reference substance solution, inject liquid chromatograph, adjust detection sensitivity; Get need testing solution and reference substance solution, inject liquid chromatograph respectively, record chromatogram Figu...
Embodiment 3
[0053] A method for HPLC method to measure silodosin impurity, described method comprises the steps:
[0054] S1. Preparation of system suitability solution: take a silodosin sample, expose to light, add methanol to the irradiated silodosin sample, and shake well to obtain a system suitability solution;
[0055] S2, preparation of reference substance solution: add methanol to the silodosin reference substance, shake well to obtain the reference substance solution;
[0056] S3, preparing the test product solution: add methanol to the silodosin test product, shake up to obtain the test product solution;
[0057] S4. Chromatographic determination: take the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram Figure I Get reference substance solution, inject liquid chromatograph, adjust detection sensitivity; Get need testing solution and reference substance solution, inject liquid chromatograph respectively, record chromatogram Figu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Illumination | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com