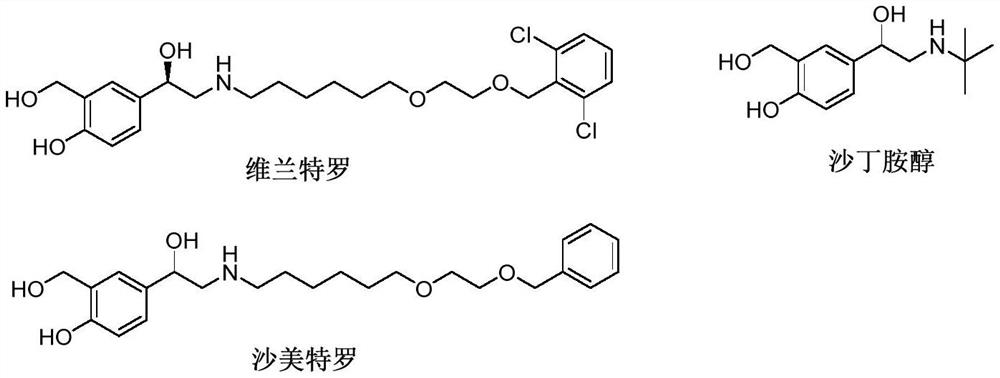
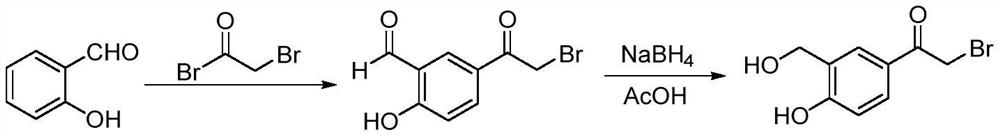
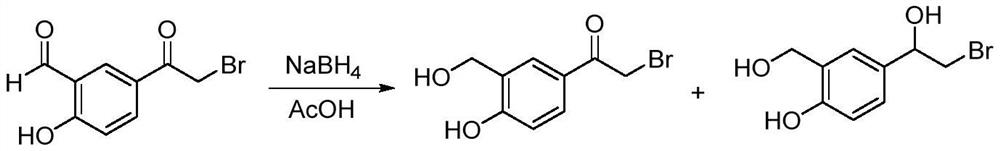
A kind of method for synthesizing vilanterol intermediate in mixed solvent
A technology of mixed solvents and intermediates, which is applied in the field of drug synthesis, can solve the problems of poor selectivity and achieve the effects of improving selectivity, simplifying the difficulty of post-processing, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 5-bromoacetyl-2-hydroxybenzaldehyde (200g) into a 5L three-necked flask, add 1,2-dichloroethane (1.5L), propionic acid (500mL), start stirring, and cool down to 5-10°C , within half an hour, add sodium borohydride (21.8g) in small amounts in batches. After the addition is complete, the liquid phase is controlled in the middle, and the remaining raw materials are less than 1%. Add 1L of water to the reaction system, stir at 0-5°C for 1 hour, and filter , washed with 500mL of water and dried to obtain 185g of white solid with a yield of 92% and a purity of 98%, of which over-reduction products accounted for 1.2%.
Embodiment 2
[0024] Add 5-bromoacetyl-2-hydroxybenzaldehyde (200g) into a 5L three-necked flask, add propionic acid (500mL), tetrahydrofuran (1.5L), start stirring, cool down to 5-10°C, within half an hour, a small amount of . Add sodium borohydride (21.8g) in batches. After the addition is complete, the liquid phase is controlled in the middle, and the remaining raw materials are less than 1%. Add 1L of water to the reaction system, stir at 0-5°C for 1 hour, filter, wash with 500mL of water, and dry. 190 g of white solid was obtained with a yield of 94% and a purity of 97.3%, of which over-reduced products accounted for 1.9%.
Embodiment 3
[0026] Add 5-bromoacetyl-2-hydroxybenzaldehyde (200g) into a 5L three-necked flask, add methanol (650mL), 1,2-dichloroethane (1.35L), start stirring, cool down to 5-10°C, Within half an hour, add sodium borohydride (21.8g) in small amounts in batches. After the addition is complete, the liquid phase is controlled in the middle, and the remaining raw materials are less than 1%. Add 1L of water to the reaction system, stir at 0-5°C for 1 hour, and filter. Washed with 500mL of water and dried to obtain 181g of white solid with a yield of 90% and a purity of 98%. Among them, over-reduction products accounted for 1.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com