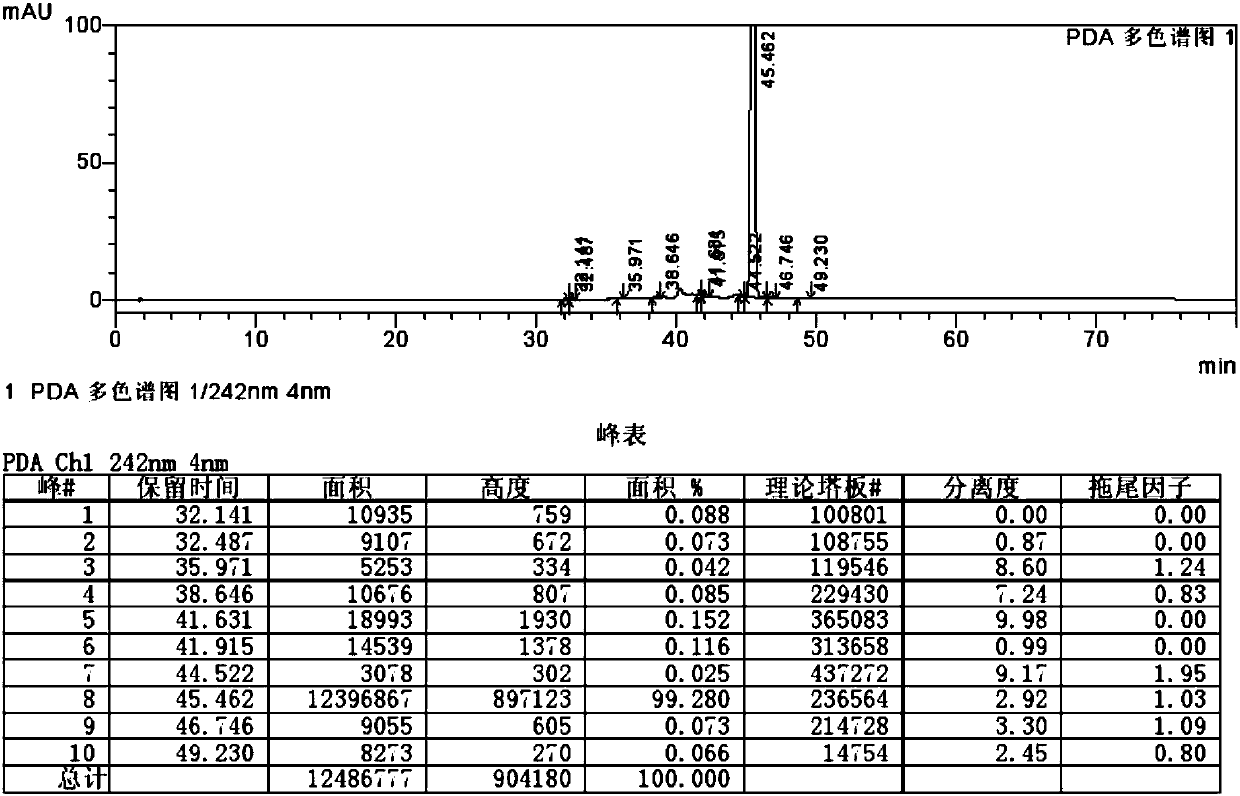
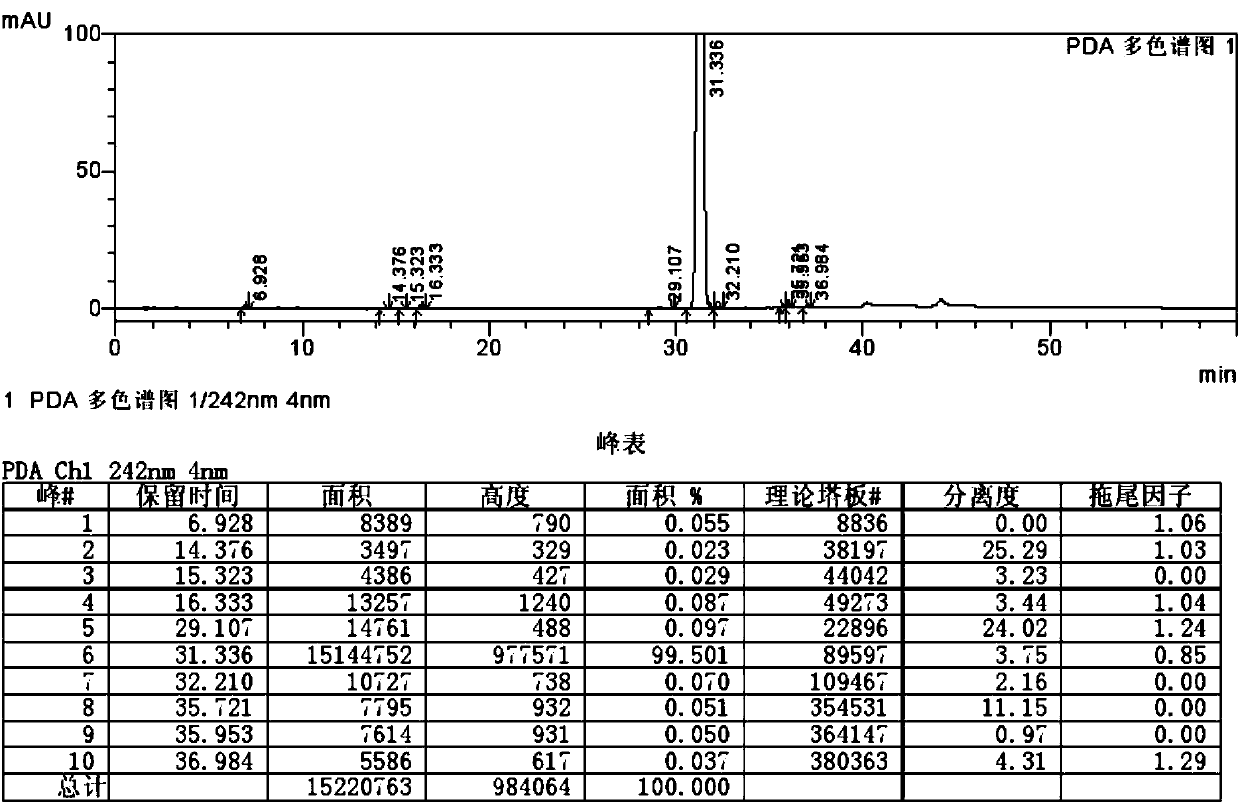
Ticagrelor impurity preparation method
A technology for ticagrelor and impurities, which is applied in the field of preparation of ticagrelor impurities, can solve the problems of low preparation efficiency and few reports on preparation methods of impurities, and achieves high chromatographic purity, easy separation and purification, and short synthesis route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 6-Chloro-N4-((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)-2-(propylthio)pyrimidine-4,5-diamine (Compound III) synthesis
[0039](1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine (R)-mandelate (compound II, 3.31g, 10mmol) and 4,6-dichloro-2-(propylthio Base)-5-aminopyrimidine (Compound I, 2g, 13mmol) was placed in a 50mL three-necked flask, and ethylene glycol (10mL) and triethylamine (4.24g, 42mmol) were added. The reaction mixture was heated to 100°C and stirred for 7 hours. Turn off the heating and cool down to 40°C naturally. Isopropyl acetate (10 mL) and water (10 mL) were added and stirred for 1 hour. Stop stirring, cool to room temperature, and let stand for liquid separation. The organic phase was separated, washed with water (10 mL), and dried over anhydrous sodium sulfate for 30 minutes. Suction filtration, the desiccant was filtered off, and the residue was separated by silica gel column chromatography (200-300 mesh column chromatography silica gel, eluent 0-5...
Embodiment 2
[0041] 7-Chloro-3-((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)-5-(propylthio)-3H-[1,2,3]triazolo Synthesis of [4,5-d]pyrimidine (Compound IV)
[0042] Compound III (2.6g, 7mmol) was placed in a 50mL single-necked bottle, toluene (10mL) and acetic acid (0.5g) were added, stirred to dissolve, and cooled to 5°C. A solution of sodium nitrite (0.8g) in water (5mL) was added dropwise, and the internal temperature did not exceed 10°C during the dropwise addition. After the addition was complete, the reaction system continued to react at 5-10°C for 1 hour. After the reaction was complete, a solution of potassium carbonate (2.8 g) in water (5 mL) was added dropwise. Stir for 15 minutes. Stop stirring, let stand for liquid separation, discard the aqueous phase, and use the organic phase directly for the next reaction.
Embodiment 3
[0044] (2-(((3aR,4S,6R,6aS)-6-((3-((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)-5-(propylthio Base)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-7-yl)amino)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][ Synthesis of 1,3]dioxol-4-yl)oxy)ethanol (impurity E)
[0045] 2-(((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxan-4-yl)oxy base) ethanol L-(+)-tartrate (compound V, 2.75g, 7.5mmol), potassium carbonate (2.47g, 18mmol) were placed in water (10mL), and stirred. This mixture was added to the toluene solution from step 2 at 5°C. After the addition was complete, the reactants were reacted at 20°C for 1 hour. Stop stirring and let stand to separate the liquid. The organic phase was washed twice with saturated sodium chloride solution (10 mL) added with 0.2 mL of acetic acid, then washed twice with saturated sodium chloride solution (10 mL), and dried over anhydrous sodium sulfate for 30 minutes. Suction filtration, filter out desiccant. The residue was evaporated t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com