Novel preparation method for florfenicol crystal
A florfenicol, a new type of technology, applied in the field of new preparation of florfenicol crystals, can solve problems such as difficult large-scale application, inconvenient application of injections, unreliable safety, etc., to achieve enhanced collision and ultra- Miniaturization effect, excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
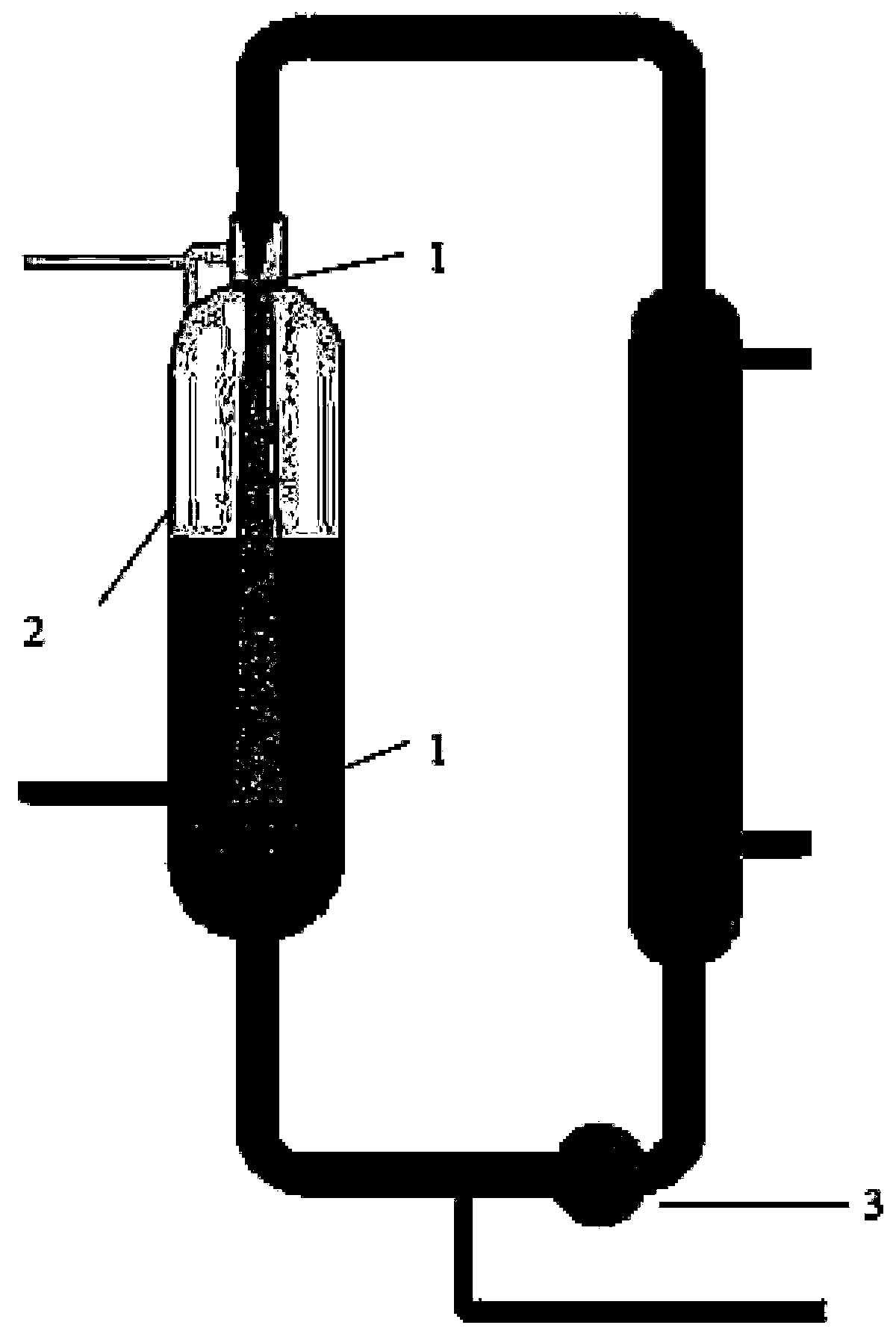
[0028] (1) In the reaction kettle 2, add solvent tetrahydrofuran, dissolve the former powder of florfenicol in tetrahydrofuran, and prepare a tetrahydrofuran solution of florfenicol, wherein the concentration of florfenicol is 30mg / ml.
[0029] (2) In another reaction kettle 2, an anti-solvent aqueous solution is prepared, which contains ethanol at a concentration of 5% and sodium hyaluronate at a concentration of 0.5%.
[0030] (3) The anti-solvent aqueous solution in step 2 is started with strong stirring, controlled at a temperature of -20 ° C, and the tetrahydrofuran solution of florfenicol is quickly pumped into the liquid surface of the anti-solvent aqueous solution through the high-pressure delivery pump 3. The opposite jet outlet 1 and the flow velocity at the outlet pipe is greater than 3 m / s. Continue vigorous stirring for 60 min. A suspension of florfenicol was obtained. Wherein the volume ratio of the anti-solvent aqueous solution to the tetrahydrofuran solution ...
Embodiment 2
[0033] (1) In the reaction kettle 2, add solvent tetrahydrofuran, dissolve the former powder of florfenicol in tetrahydrofuran, and prepare a tetrahydrofuran solution of florfenicol, wherein the concentration of florfenicol is 80mg / ml.
[0034] (2) In another reaction kettle 2, prepare an aqueous anti-solvent solution, which contains ethanol at a concentration of 1%; contains sodium hyaluronate 0.1%.
[0035] (3) Turn on the strong stirring of the anti-solvent aqueous solution in step 2, and control the temperature at 0°C, and quickly pump the tetrahydrofuran solution of florfenicol into the liquid surface of the anti-solvent aqueous solution through the high-pressure delivery pump 3. Type jet outlet 1 and the flow velocity at the outlet pipe is greater than 3 m / s. Continue vigorous stirring for 30 min. A suspension of florfenicol was obtained. Wherein the volume ratio of the anti-solvent aqueous solution to the tetrahydrofuran solution of Florfenicol is 50:1.
[0036] (4) ...
Embodiment 3
[0038] (1) In reaction kettle 2, add solvent tetrahydrofuran, dissolve the former powder of florfenicol in tetrahydrofuran, prepare the tetrahydrofuran solution of florfenicol, wherein the concentration of florfenicol is 50mg / ml.
[0039] (2) In another reaction kettle 2, an anti-solvent aqueous solution is prepared, which contains ethanol at a concentration of 3%; contains sodium hyaluronate 0.3%.
[0040] (3) The anti-solvent aqueous solution in step 2 is started to stir vigorously, controlled at a temperature of -10°C, and the tetrahydrofuran solution of florfenicol is quickly pumped under the liquid surface of the anti-solvent aqueous solution through the high-pressure delivery pump 3. The opposite jet outlet 1 and the flow velocity at the outlet pipe is greater than 3 m / s. Continue vigorous stirring for 60 min. A suspension of florfenicol was obtained. Wherein the volume ratio of the anti-solvent aqueous solution to the tetrahydrofuran solution of Florfenicol is 25:1. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com