A method for purifying copper electrolyte by two removals and two accumulations
A copper electrolyte solution and a technology for purifying copper, applied in the field of copper electrolytic refining, can solve problems such as the generation of toxic hydrogen arsenide gas, poor impurity open circuit, poor working environment, etc., and achieve the effects of good arsenic removal, improved environmental protection, and increased income.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
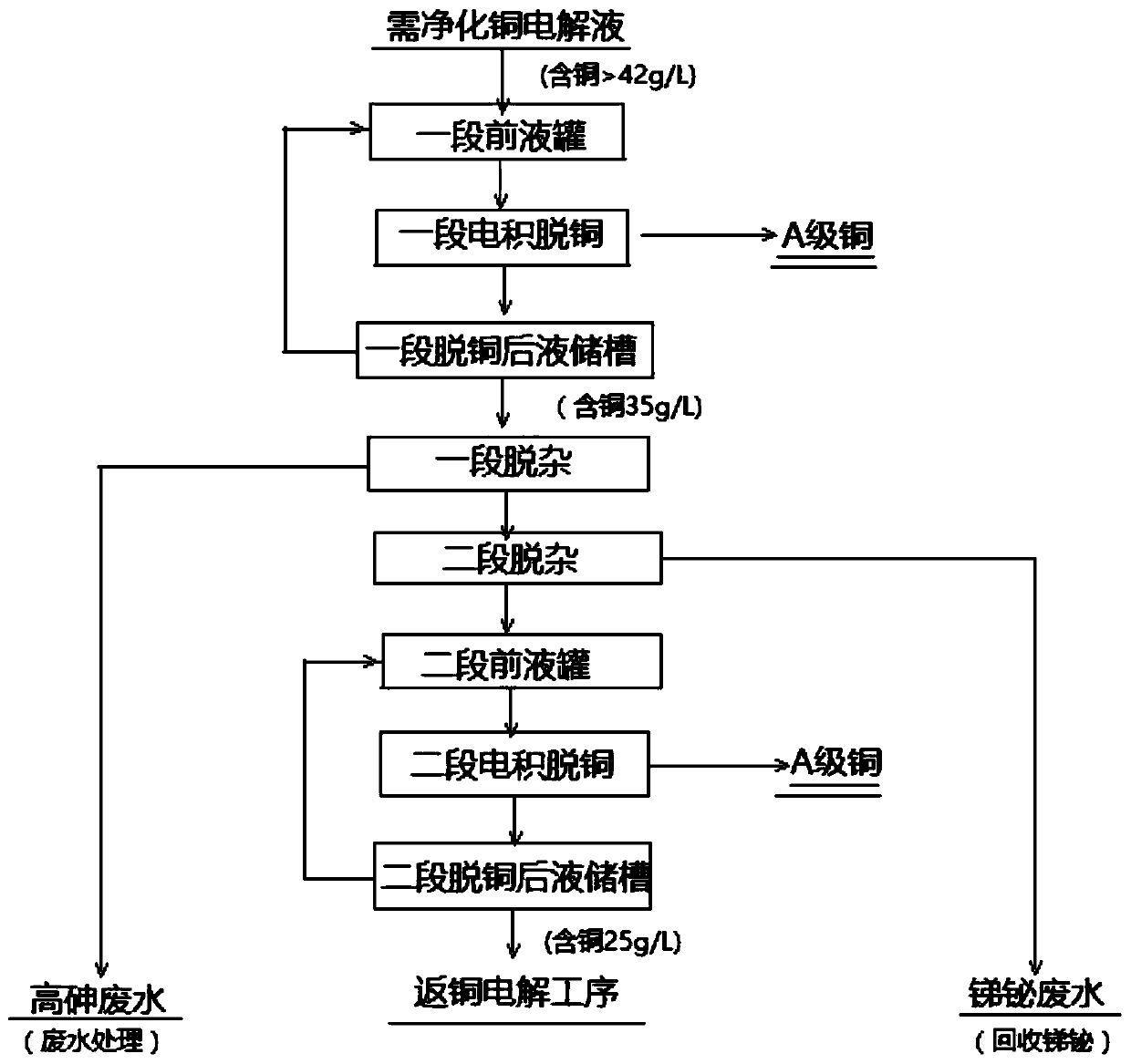
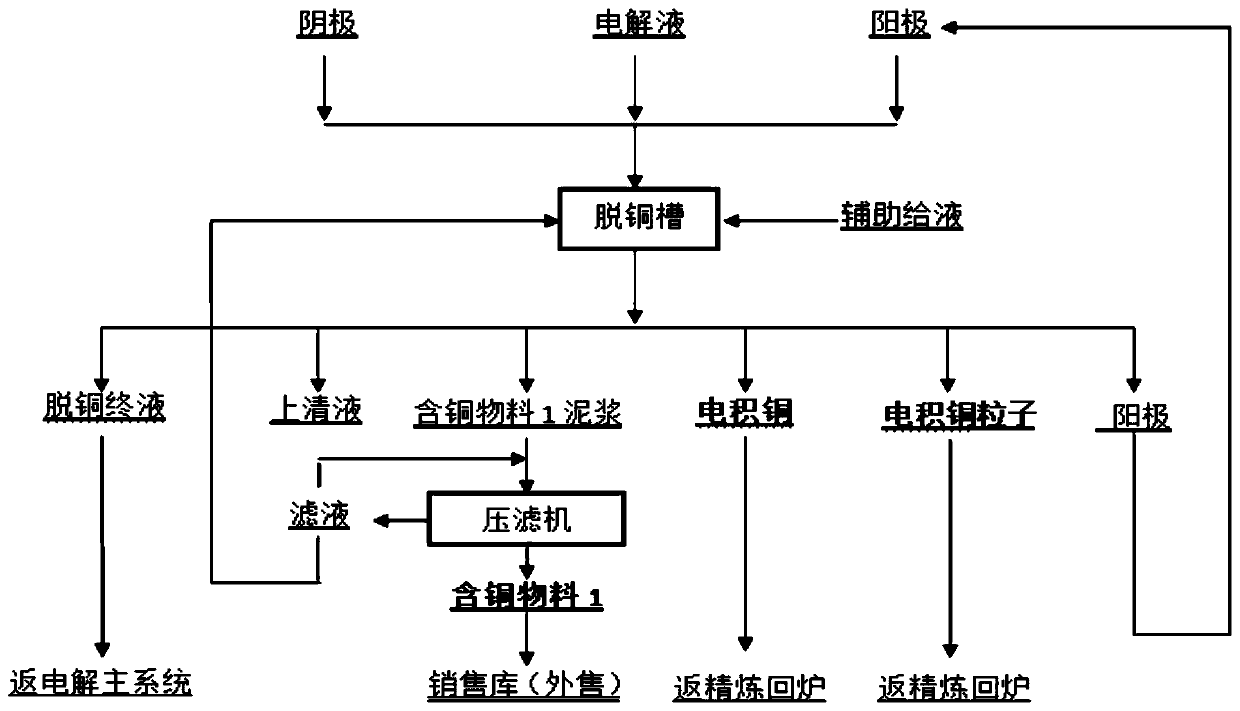
[0049] (1) One-stage electrowinning copper removal
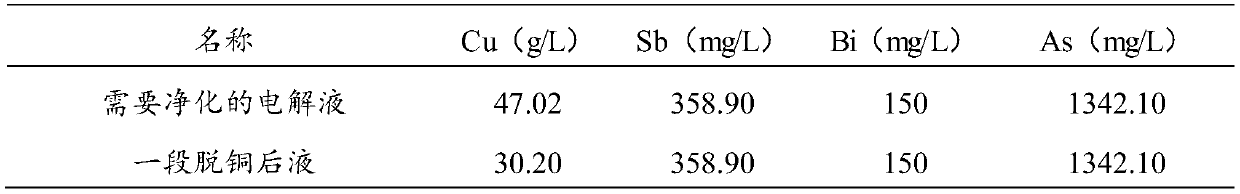
[0050] The electrolytic solution containing 47.02g / L of copper that needs to be purified (see Table 1 for composition) is pumped to the first section of the front liquid cylinder, and the electrolyte in the first section of the front liquid cylinder is heated to 65°C by plate exchange, and then given to the bottom of the first section of electrowinning tank. Liquid, single-end liquid return for one stage of decopper, after one stage of copper removal, the liquid pumps to a stage of impurity removal process. When the concentration of copper ions in the solution drops from 47.02g / L to 30.2g / L after a period of copper removal, the electrowinning is stopped and the cathode produces A-grade copper.
[0051] Wherein, the process parameters of the first stage of electrowinning copper removal include: the number of slots for each group of cathode plates is 280; the current is 13000A; the current intensity is 280A / m 2 ; The electric...
Embodiment 2
[0072] (1) One-stage electrowinning copper removal
[0073] The electrolytic solution containing 48.20g / L of copper that needs to be purified (see Table 5 for composition) is pumped to the first section of the front liquid cylinder, and the electrolyte in the first section of the front liquid cylinder is heated to 65°C by plate exchange, and then given to the bottom of the first section of electrowinning tank. Liquid, single-end liquid return for one stage of decopper, after one stage of copper removal, the liquid pumps to a stage of impurity removal process. When the concentration of liquid copper ions drops from 48.20g / L to 29.82g / L after a period of copper removal, the electrowinning is stopped and the cathode produces A-grade copper.
[0074] Wherein, the process parameters of the first stage of electrowinning copper removal include: the number of slots for each group of cathode plates is 280; the current is 13000A; the current intensity is 280A / m 2 ; The electric efficie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com