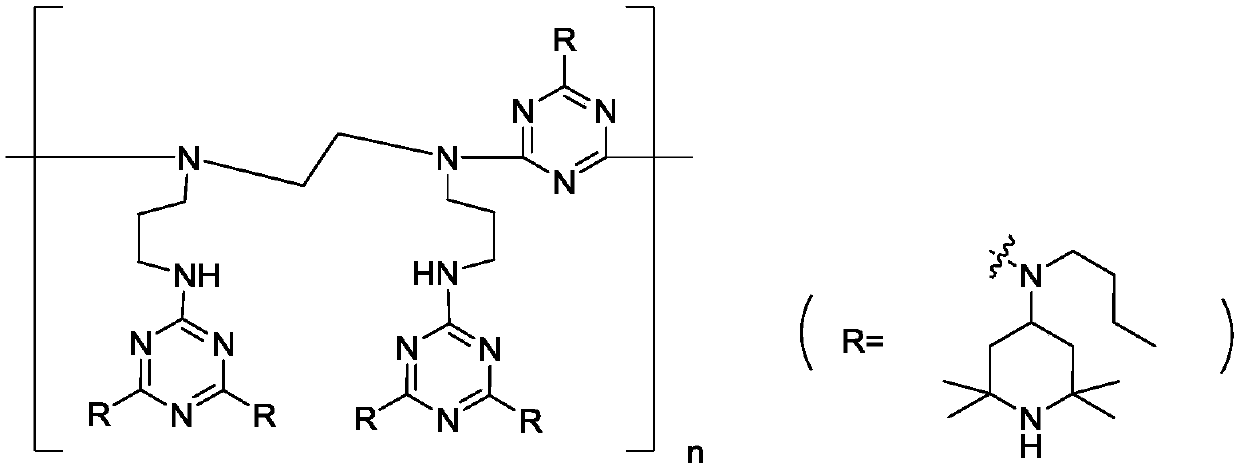
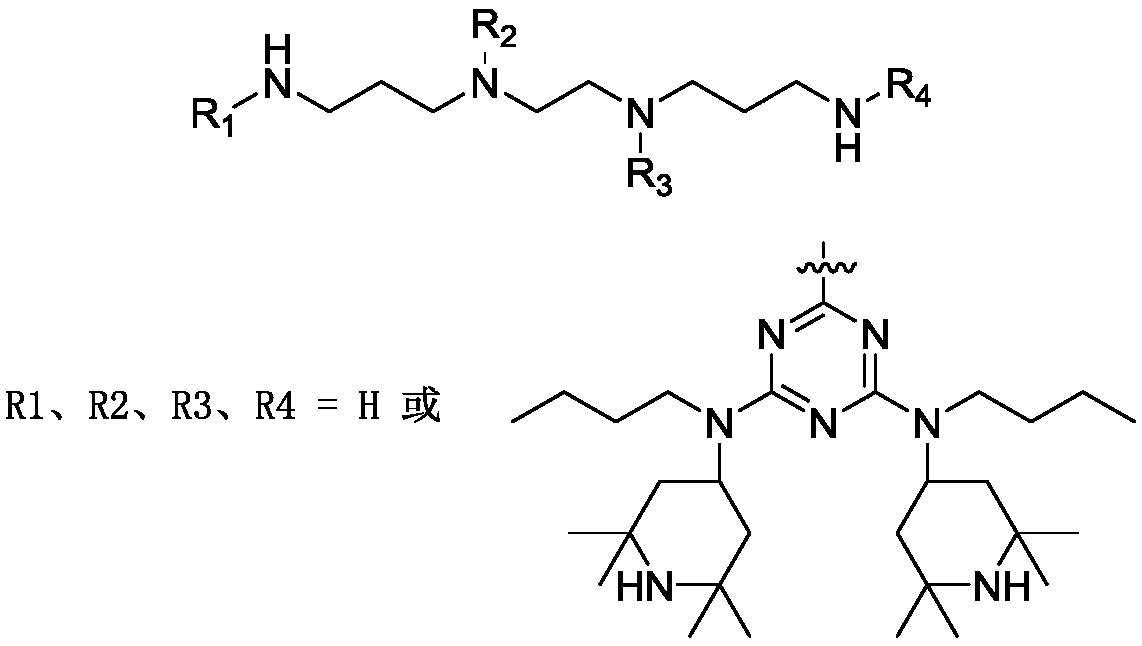
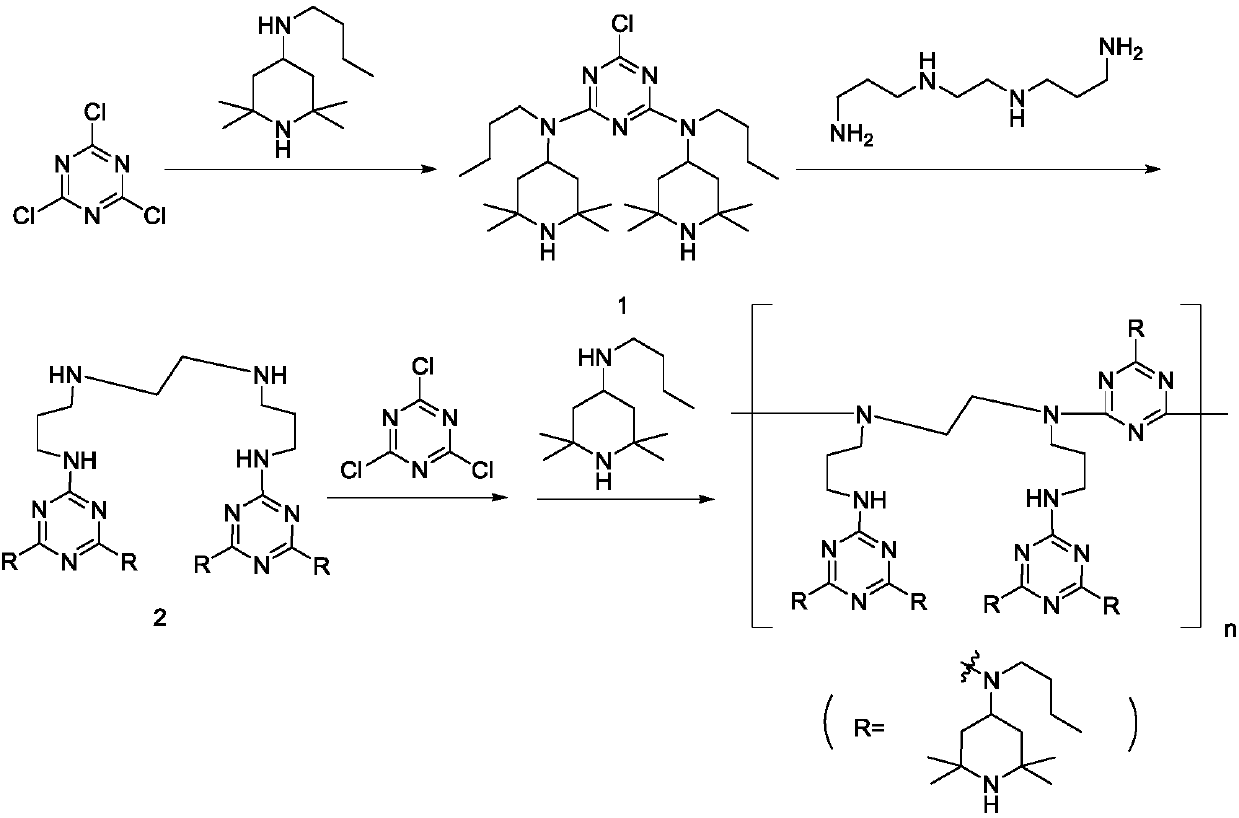
Polymeric hindered amine light stabilizer HA-88 and preparation method of polymeric hindered amine light stabilizer HA-88 intermediate
A hindered amine light stabilizer, HA-88 technology, applied in the direction of organic chemistry, can solve the problems of adverse effects of HALS light stabilization effect, achieve outstanding application effect, improve molecular weight distribution, and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Disperse 1.84g (10mmol) cyanuric chloride in 20mL toluene, add 4.46g (21mmol) N-butyl-2,2,6,6-tetramethyl-4-piperidinamine dropwise to the system at room temperature After the dropwise addition, add 1.17g of sodium carbonate, heat up to 45°C for reaction, monitor the reaction by HPLC, the content of N-butyl-2,2,6,6-tetramethyl-4-piperidinamine is less than 0.5% The reaction is considered to be complete. After the reaction, the reaction solution was slowly added dropwise to a solution of 0.85 g (4.9 mmol) N,N'-bis(3-aminopropyl)ethylenediamine in 10 mL of toluene. After the dropwise addition, 0.58 g of sodium carbonate was added, and refluxed for dehydration reaction. The reaction was monitored by HPLC, and the reaction was considered as complete when the content of the reaction product in the first step was less than 0.5%. The content of disubstitutes in the intermediate is 87.0%.
[0040] This embodiment also provides a method for preparing HA-88 by using the prepar...
Embodiment 2
[0044] Disperse 1.84g (10mmol) cyanuric chloride in 20mL toluene, add 4.36g (20.5mmol) N-butyl-2,2,6,6-tetramethyl-4-piperidine dropwise to the system at room temperature After completion of the amine, add 2.4mL 30% potassium hydroxide aqueous solution dropwise, heat up to 45°C for reaction, monitor the reaction by HPLC, the content of N-butyl-2,2,6,6-tetramethyl-4-piperidinamine When it is less than 0.5%, the reaction is considered to be over. After the reaction, the reaction solution was slowly added dropwise to a solution of 0.91 g (5.2 mmol) N,N'-bis(3-aminopropyl)ethylenediamine in 10 mL toluene. After completion, 1.2 mL of 30% potassium hydroxide aqueous solution was added dropwise, and refluxed for dehydration reaction. The reaction was monitored by HPLC, and the reaction was considered as complete when the content of the reaction product in the first step was less than 0.5%. The content of disubstitute in the intermediate is 91.1%.
[0045] This embodiment also prov...
Embodiment 3
[0049] Disperse 1.84g (10mmol) of cyanuric chloride in 20mL of xylene, and drop 4.36g (20.5mmol) of N-butyl-2,2,6,6-tetramethyl-4-piperol into the system at room temperature 10mL xylene solution of pyridineamine, after completion, add 2.2mL 30% sodium hydroxide aqueous solution dropwise, heat up to 45°C for reaction, monitor the reaction by HPLC, N-butyl-2,2,6,6-tetramethyl-4 - When the content of piperidinamine is lower than 0.5%, the reaction is considered as complete. After the reaction, the reaction solution was slowly added dropwise to a solution of 0.91 g (5.2 mmol) N,N'-bis(3-aminopropyl)ethylenediamine in 10 mL xylene. After completion, 1.1 mL of 30% sodium hydroxide aqueous solution was added dropwise, and the reaction was refluxed. The reaction was monitored by HPLC, and the reaction was considered as complete when the content of the reaction product in the first step was less than 0.5%. The content of disubstitutes in the intermediate is 82.6%.
[0050] This embo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com