Method for synthesizing 4-alkylresorcinol through solvent-free system
A technology of alkyl resorcinol and acyl resorcinol, which is applied in the field of solvent-free synthesis of 4-alkylresorcinol, can solve problems such as unfavorable environmental protection, achieve waste reduction, mild reaction conditions, and reduce environmental protection cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
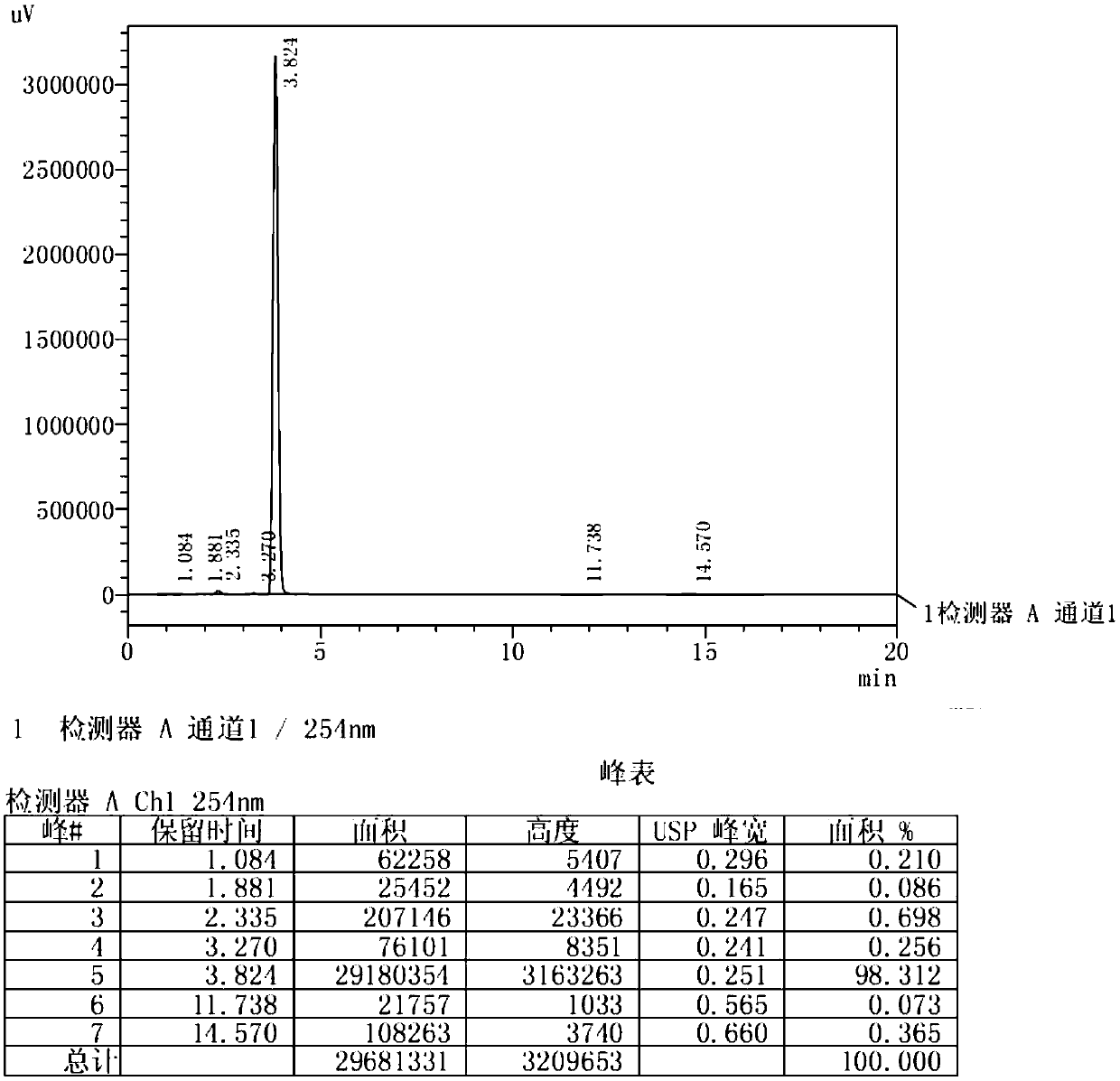
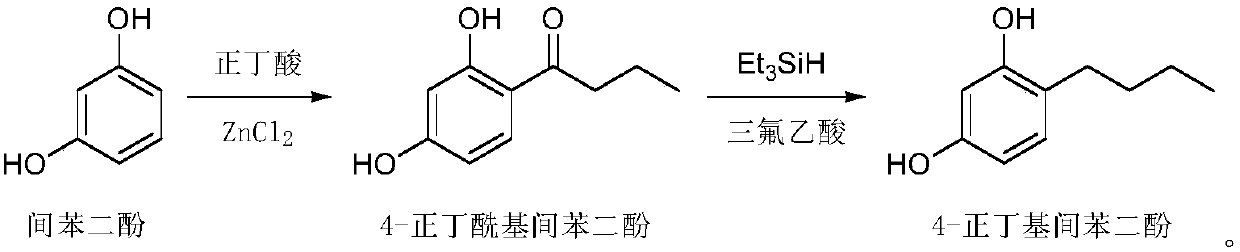
[0030] (1) Dissolve raw materials resorcinol (500g, 4.54mol) and zinc chloride (804g, 5.9mol) in n-butyric acid (600g, 6.81mol), heat to 100°C and stir. After the reaction of the raw materials is complete, the reaction solution is cooled and added to water. After stirring, a large amount of solids are precipitated, filtered and washed with water. After the solids are dried, recrystallized to obtain 4-n-butyryl resorcinol (724g, figure 1 , HPLC: 98.3%);
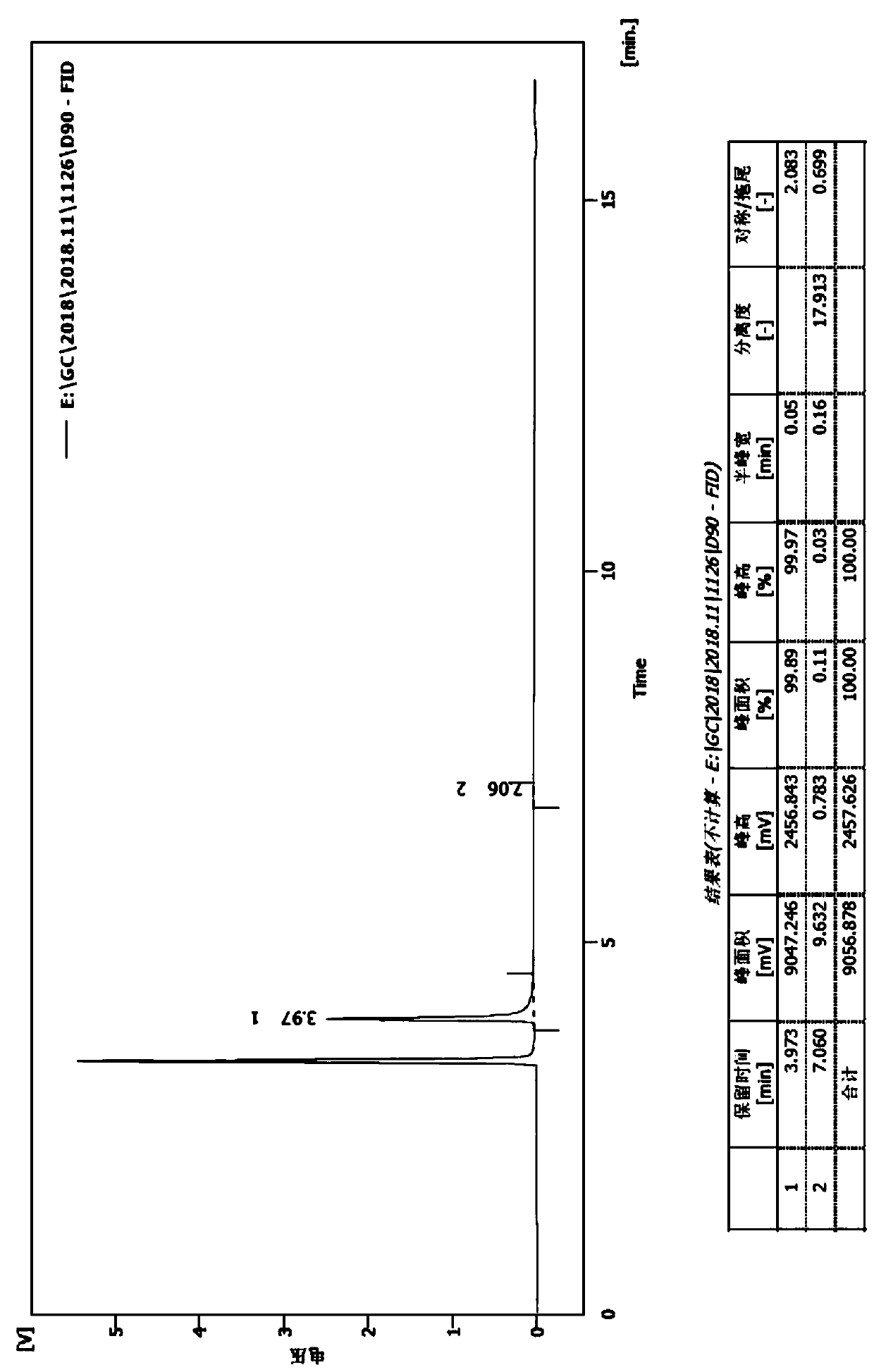
[0031] (2) Dissolve 4-n-butyryl resorcinol (724g, 4.02mol) in trifluoroacetic acid (2.29kg, 20.1mol), cool in an ice-water bath to 0°C, add triethylsilane (1.4kg, 12.06mol), after the addition, the reaction solution was heated to 60°C and stirred overnight. After the reaction of the raw materials is complete, the reaction solution is poured into water to quench, and after continuous stirring, the layers are separated, the organic layer is extracted with ethyl acetate, the combined organic phase is washed with saturated brine,...
Embodiment 2
[0033] (1) The raw material resorcinol (500g, 4.54mol) and zinc chloride (618g, 4.54mol) were dissolved in n-butyric acid (400g, 4.54mol), heated to 80°C and stirred. After the reaction of the raw materials was complete, the reaction solution was cooled and added to water. After stirring, a large amount of solids were precipitated, filtered and washed with water. The obtained solids were dried and recrystallized to obtain 4-n-butyryl resorcinol (561 g);
[0034] (2) Dissolve 4-n-butyryl resorcinol (561g, 3.1mol) in trifluoroacetic acid (707g, 6.2mol), cool in an ice-water bath to 0°C, add triethylsilane (721g, 6.2mol) dropwise ), after the addition, the reaction solution was heated to 40°C and stirred overnight. After the reaction of the raw materials is complete, the reaction solution is poured into water to quench, and after continuous stirring, the layers are separated, the organic layer is extracted with ethyl acetate, the combined organic phase is washed with saturated br...
Embodiment 3
[0036](1) The raw material resorcinol (500g, 4.54mol) and zinc chloride (1.24kg, 9.08mol) were dissolved in n-butyric acid (800g, 9.08mol), heated to 120°C and stirred. After the reaction of the raw materials was complete, the reaction solution was cooled and added to water. After stirring, a large amount of solids were precipitated, filtered and washed with water. The obtained solids were dried and recrystallized to obtain 4-n-butyryl resorcinol (602 g);
[0037] (2) Dissolve 4-n-butyryl resorcinol (602g, 3.34mol) in trifluoroacetic acid (3.81kg, 33.4mol), cool in an ice-water bath to 0°C, add triethylsilane (1.94kg, 16.7mol), after the addition, the reaction solution was heated to 80°C and stirred overnight. After the reaction of the raw materials is complete, the reaction solution is poured into water to quench, and after continuous stirring, the layers are separated, the organic layer is extracted with ethyl acetate, the combined organic phase is washed with saturated brin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com