Prostatic cancer diagnosis, classification or prognosis marker, detection reagent or kit, system and application thereof
A prognostic marker, prostate cancer technology, applied in the field of medical diagnosis, can solve the problems of low specificity, imperfect PSA, poor sensitivity and specificity of prostate cancer, etc., and achieve a good indicator effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] This embodiment provides a method for detecting the concentration ratio of serum exosome-derived GCP2 to exosome-derived CD63, including:
[0052] 1. Establishment of the basic detection method: use the exosome extraction kit (QIAGEN: exoEasy Maxi Kit or miRCURY Exosome Kits; Thermofisher: Total Exosome Isolation Reagent fromserum) to extract the total exosomes in the serum and dissolve them in In the PBS solution, an exosome-PBS solution was formed. The GCP2 and CD63 protein concentrations in the exosome-PBS solution were detected with an ELISA kit (the ELISA kit for detecting GCP2 is Human PSMA / FOLH1 DuoSet ELISA from R&DSystem; the ELISA kit for detecting CD63 is LifespanbiosciencesHuman CD63 ELISA Kit Sandwich ELISA), wherein 100 μL exosome-PBS solution was used for the detection of GCP2, and 100 μL exosome-PBS solution was used for CD63. The ratio of GCP2 concentration to CD63 concentration was calculated.
[0053] 2. Optimize the amount of PBS solution used to d...
Embodiment 2
[0057] This embodiment provides a particle number ratio of serum GCP2 positive exosomes to CD63 positive exosomes (F exo / T exo ) detection methods, including:
[0058] 1. Establishment of the basic detection method: use an exosome extraction kit (QIAGEN: exoEasy Maxi Kit or miRCURY Exosome Kits; Thermofisher: Total Exosome Isolation Reagent fromserum) to extract the total exosomes in the serum and dissolve them in In the PBS solution, an exosome-PBS solution was formed.
[0059] Incubate CD63 antibody with magnetic beads overnight at 4°C to form CD63 magnetic bead solution. Then 200 μL of exosome-PBS solution was diluted 100 times to 20 ml and 500 μL of exosome dilution was taken from 20 ml. Add 50 μL CD63 magnetic bead solution to 500 μL exosome dilution solution and incubate at 37°C for 1 hour. Place the above-incubated mixture on a magnetic stand to precipitate the magnetic beads at the bottom of the eppendorf centrifuge tube. After aspirating the supernatant, rinse t...
Embodiment 3
[0063] The purpose of this example is to evaluate the effect of the concentration ratio of serum exosome-derived GCP2 and exosome-derived CD63 on the diagnosis of prostate cancer:
[0064] 1. Enrollment samples: 30 subjects with clinical diagnosis results and known serum PSA concentration were selected as enrollment samples, and the specific information is shown in Table 2.
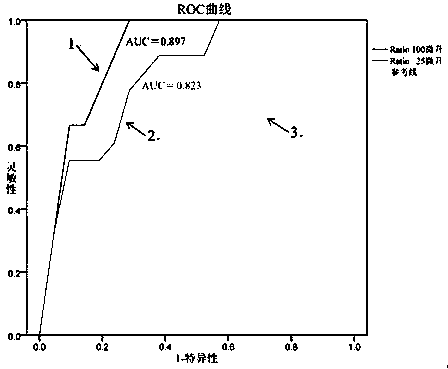
[0065] 2. Detect the concentration ratio of serum exosome-derived GCP2 and serum exosome-derived CD63 in the incoming sample (see Example 1 for the specific method, dissolve the exosome extract of 1mL serum sample in 50μL PBS solution, divide into 25 μL (i.e. ratio 25 μL group), used to detect GCP2 or CD63; or, the exosome extract of 1 mL serum sample was dissolved in 200 μL PBS solution, and divided into 100 μL (i.e. ratio 100 μL group), used to detect GCP2 or CD63), And draw the ROC curve according to the known diagnostic results and the detected concentration ratio. The result of described concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com