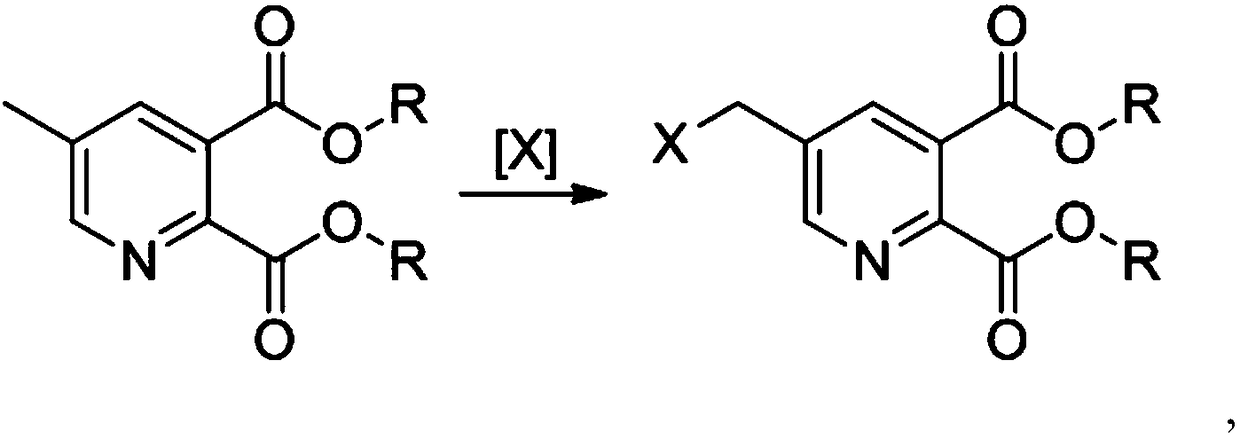
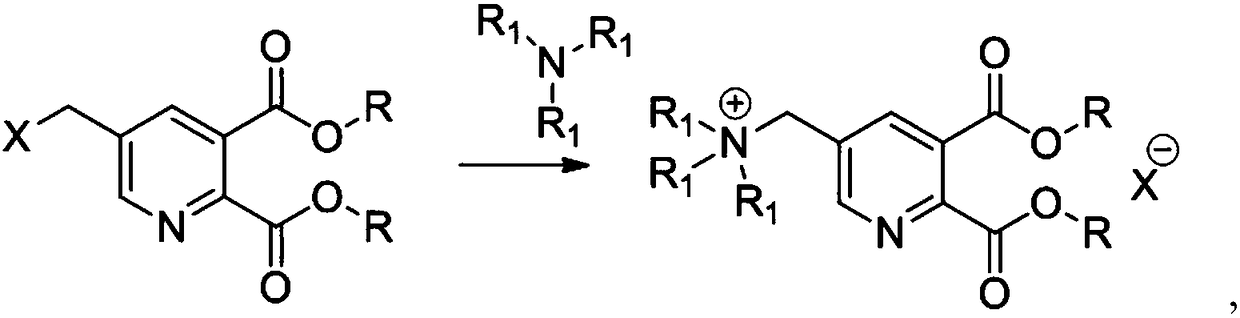
Preparation method for substituted pyridine dicarboxylic acid derivative
A technology of dipicolinic acid and derivatives, which is applied in the field of preparation of substituted dipicolinic acid derivatives, can solve the problems of a large number of waste liquids, harsh process conditions, complicated preparation processes, etc., and achieves the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment provides a preparation method of substituted dipicolinic acid derivatives, comprising the following preparation steps:
[0049] (1) Put 0.418mol 5-methylpyridine-2,3-dicarboxylate diethyl into 2.090mol 1,2-dichloroethane, start stirring, heat up to 80°C, add 0.004mol azobisiso Mix butyronitrile and 0.250mol bromine, control the same temperature and add bromine dropwise to the 1,2-dichloroethane solution of 5-methylpyridine-2,3-dicarboxylate, keep warm after the dropwise addition After 2 hours, the temperature was lowered to obtain a solution of 5-bromomethylpyridine-2,3-dicarboxylic acid diethyl ester;
[0050](2) Transfer the solution of diethyl 5-bromomethylpyridine-2,3-dicarboxylate into a pressure vessel, feed trimethylamine gas to 6 atm, start stirring, keep at 20°C for 5 hours, and add additional Trimethylamine keeps pressure 6atm, and reaction finishes, is distilled to pH=7, adds 183 gram waters, layering, organic phase continues to be used in st...
Embodiment 2
[0053] (1) Add 0.418mol 5-methylpyridine-2,3-dicarboxylate to 12.540mol chlorobenzene, start stirring, heat up to 100°C, mix 0.021mol dimethyl azobisisobutyrate and 0.418 mol bromosuccinimide is mixed, and bromosuccinimide is put into the chlorobenzene solution of 5-methylpyridine-2,3-dicarboxylate at the same temperature, and the reaction is kept for 6 hours after the addition is completed. , cooling to obtain a solution of 5-bromomethylpyridine-2,3-dicarboxylic acid dimethyl ester;
[0054] (2) Transfer the solution of dimethyl 5-bromomethylpyridine-2,3-dicarboxylate into a pressure vessel, feed trimethylamine gas to 4.5atm, start stirring, keep 35°C for 3.5 hours, and make up the reaction process Add trimethylamine to keep the pressure at 4.5atm, the reaction is over, distill to pH=9, add 40 grams of water, separate layers, the organic phase continues to be used for step (1) synthesis, and obtains 134 grams of water phase, which is the 70% quaternary ammonium made Saline s...
Embodiment 3
[0057] (1) Add 0.418mol 5-methylpyridine-2,3-dicarboxylate into 7.106mol chloroform, start stirring, heat up to 60°C, mix 0.012mol azobisisobutylimidazoline hydrochloride and 0.335 mol of sodium hypochlorite was mixed, and sodium hypochlorite was added dropwise into the chloroform solution of 5-methylpyridine-2,3-dicarboxylate under control of the same temperature. , a chloroform solution of dimethyl 3-dicarboxylate;
[0058] (2) Transfer the solution of dimethyl 5-chloromethylpyridine-2,3-dicarboxylate into a pressure vessel, add triethylamine, start stirring, keep at 120°C for 1.5 hours, and add triethylamine during the reaction process Keep pressure 2.5atm, reaction finishes, distills to pH=8, adds 57 gram waters, layering, organic phase continues to be used in step (1) synthesis, obtains 115 gram water phases, is the 50% quaternary ammonium salt aqueous solution ( Contains quaternary ammonium salt 0.167mol);
[0059] (3) Add 1.003mol sodium hydroxide and 5.016mol methano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com