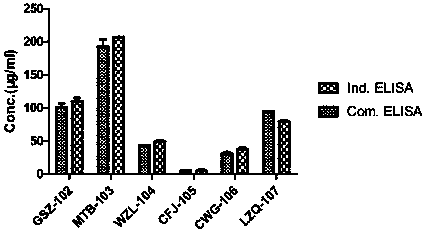Method and kit for detecting blood-drug concentration of PD-1 antibody
A technology of PD-1 and blood drug concentration, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem of high detection background and achieve good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Material
[0042] 1.1 Drugs and reagents: PD-1, biotinylated anti-PD-1 (biotin-labeled PD-1 antibody); standard: PD-1 monoclonal antibody, 200mg; coating solution: 1.6g Na 2 CO 3 , 2.9g NaHCO 3 , 0.5g NaN 3 , Add double-distilled water to 1L, adjust the pH to 9.6, filter, aliquot and store at 4℃; PBS: 0.2722g KH2PO4, 3.58g Na2HPO4.12H2O, 8.063g NaCl, 0.2066g KCl, add double-distilled water to 1L, Adjust the pH to 7.4, filter, aliquot, and store at 4°C; washing solution (PBST): add 0.05% Tween 20 to PBS. Shake well before use; blocking solution: PBST solution containing 2% BSA; sample diluent: PBST solution containing 0.5% BSA; antibody diluent: PBST solution containing 0.5% BSA; antibody: Streptavidin Protein, HRPconjugate (21126) form Thermo, use ratio 1:10000; blank serum: purchased from Shunran (Shanghai) Biotechnology Co., Ltd. Wherein, the biotinylated anti-PD-1 is prepared by the following method: the antibody concentration is controlled to between 0.8mg / ml, an...
Embodiment 2
[0095] 1. Establish the quality inspection standard of each component stock solution
[0096] According to 1.4 ELISA operation steps, the quality inspection of each component is performed separately. The quality inspection standard of each component is that the experimental data deviation (RSD) of different days is less than 20%. The stock solution that has passed the quality inspection can be used as the component in the kit. The experimental results show that the deviation of the IC50 value in different days does not exceed 20% (Table 10).
[0097] Table 10 The deviation of the stock solution components in different days
[0098]
[0099] 2. Lyophilization of kit components
[0100] The freeze-drying process is: pre-freezing temperature is -60℃, lasts 6 hours, cold trap temperature is -70℃, vacuum degree is guaranteed below 10Pa, drying time is 30 hours, and the freeze-dried product has a full appearance.
[0101] 3. Quality inspection of the kit
[0102] After lyophilization, each co...
Embodiment 3
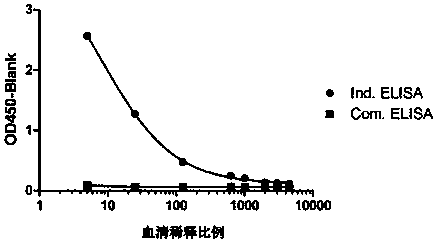
[0122] The method in this example is a general method that can detect multiple PD-1 antibodies (provided by different manufacturers) under research and on the market. The specific experiment is as follows, according to 1.4 ELISA procedure to carry out various antibody verification, the result shows ( figure 2 ), different antibody drugs may use this method for serum drug content determination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com