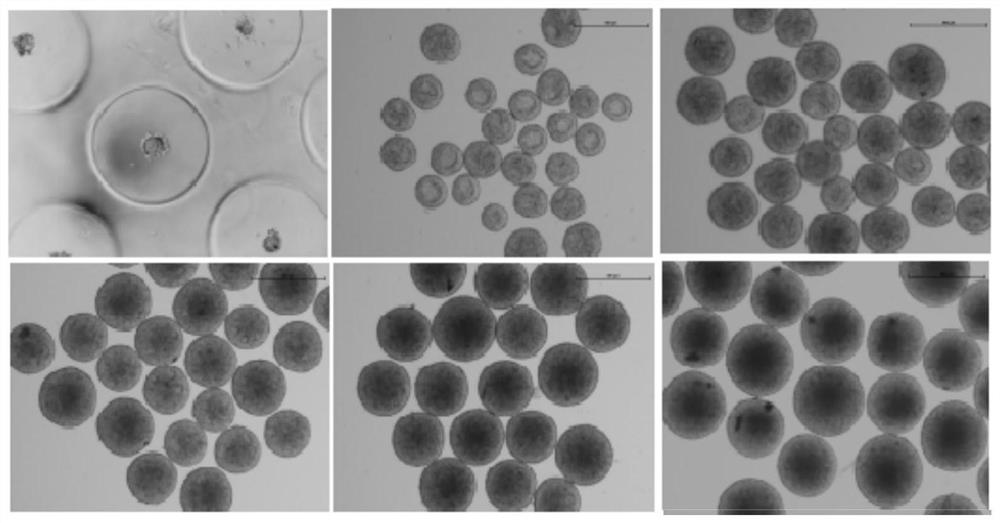
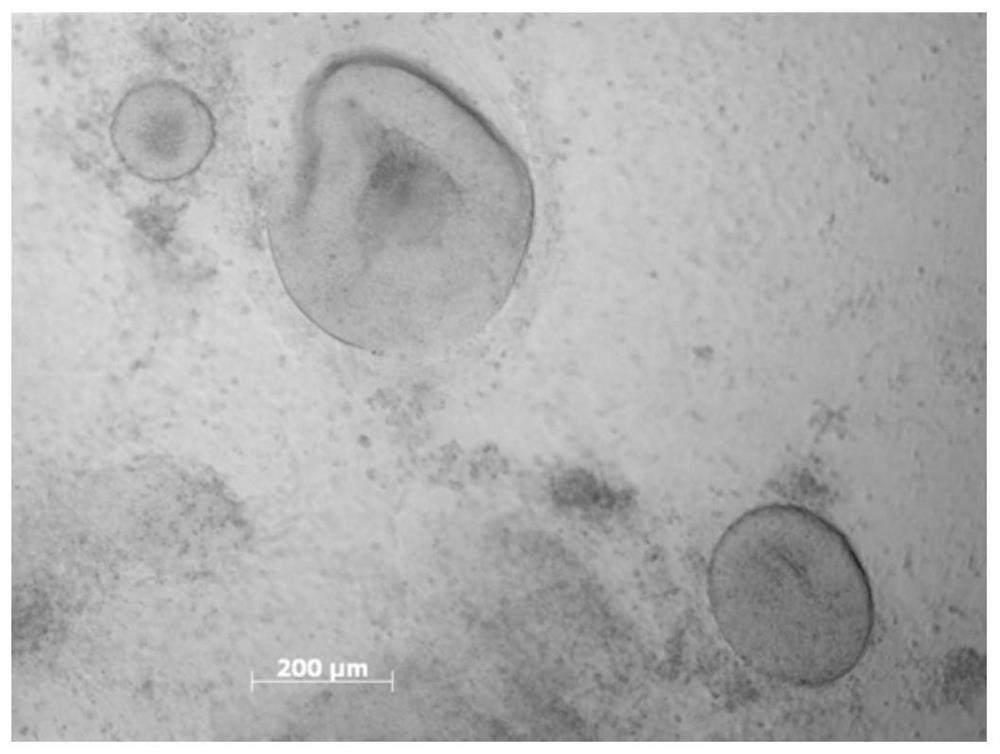
A Simple, Efficient and Mechanizable Induction Method for Differentiation of Human Pluripotent Stem Cells into Retinal Tissue
A technology of human pluripotent stem cells and retina, which is applied in the field of programmed induction, can solve the problems of poor differentiation homogeneity, poor experiment repeatability, and long learning time, and achieve the effects of good structure formation, increased acquisition, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Maintenance culture and passage of human pluripotent stem cells
[0032] ① Cells: SB cell line (hiPSC cells derived from skin fibroblasts; Cellapy, CA4002106)
[0033] ②Reagents and consumables:
[0034] 1) mTeSR1 medium: STEM CELL, #05851, 4°C
[0035] 2) EDTA: Invitrogen, 15575-038, room temperature
[0036] 3) PBS (1X): Gino Biomedical Technology Co., Ltd., 14111202, room temperature
[0037] 4) Matrigel: Corning, 354277, -20°C
[0038] 5) Six-hole plate: FALCON, 353046
[0039] 6) Centrifuge tube: BD FALCON, 352096
[0040] ③Instrument:
[0041] 1) CO 2 Incubator: SANYO, MCO-20A1C
[0042] 2) Inverted microscope: Nikon, TS100
[0043] ④ Step: hPSC cells (SB cell line) are maintained in mTeSR1 medium, and the clones can grow to occupy about 80% of the bottom area of the well in about 4-5 days. Differentiated cells were scraped off under a light microscope before passaging. Aspirate the culture medium and rinse twice with sterile PBS. Add 1ml of 0.5mM...
Embodiment 2
[0104] The most significant technical improvement of the present invention includes two points: first, a single hPSC is used as the starting cell for differentiation, and the number of cells is limited to induce differentiation; second, the expression curve of DKK-1 is increased, and DKK-1 protein is supplemented as needed, To ensure that the differentiation is suitable for most hPSC cell lines.
[0105] 1. Use a single hPSC as the starting cell for differentiation, and limit the number of cells to induce differentiation in order to realize mechanized operation
[0106] The efficiency of induction of differentiation and the number of cell populations with different fates are mainly determined by the size of embryoid bodies and the density after attachment. Therefore, the existing differentiation schemes cannot quantitatively control the size and attachment density of embryoid bodies, which seriously affects the homogeneity of their differentiation and the possibility of large-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com